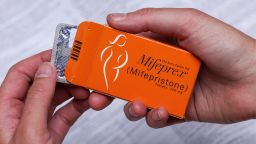
Twelve states led by liberal attorneys general announced Friday that they had sued the Food and Drug Administration, saying its limits on mifepristone, one of the two drugs used for medication abortion, are too strict.
The suit is a possible hedge by states waiting to see how a federal judge in Texas rules in a lawsuit brought by anti-abortion groups seeking to block the FDA’s approval of mifepristone altogether. Conflicting rulings could mean the Supreme Court is asked to sort out the issue.
RELATED: How a medication abortion, also known as an ‘abortion pill,’ works
“The federal government has known for years that mifepristone is safe and effective,” Washington state Attorney General Bob Ferguson said in a statement. “In the wake of the Supreme Court’s radical decision overturning Roe v. Wade, the FDA is now exposing doctors, pharmacists and patients to unnecessary risk. The FDA’s excessive restrictions on this important drug have no basis in medical science.”
Mifepristone was first approved in 2000 and medication abortion accounts for more than half of the abortions in the US. It is the first drug, followed by misoprostol, in the medication abortion regimen. Patients and providers must sign agreements stating the drug will be used to end a pregnancy, and pharmacies must have special certification.
The lawsuit was filed in federal court in the Eastern District of Washington state. The states in the lawsuit are: Washington, Oregon, Arizona, Colorado, Connecticut, Delaware, Illinois, Michigan, Nevada, New Mexico, Rhode Island and Vermont.
Final brief filed in Texas lawsuit
A lawsuit seeking to block the use of medication abortion nationwide could receive an initial decision at any moment, after the plaintiffs in the case submitted to the court on Friday their final brief on the challenge.
The lawsuit, filed in November by anti-abortion advocates against FDA, challenges the two-decade-old approval of mifepristone, the first drug in the medication abortion process.
A decision by US District Judge Matthew Kacsmaryk, an appointee of former President Donald Trump, in favor of the plaintiffs could have far-reaching consequences since medication abortion now makes up a majority of abortions obtained in the US.
In the filing submitted Friday, the anti-abortion advocates rehashed many of the arguments they made in earlier briefs. Its submission means that Kacsmaryk could soon rule on a motion by the plaintiffs to temporarily block use of the medication. The judge had previously said that once the February 24 filing deadline ended, “briefing will then be closed on the matter, absent any ‘exceptional or extraordinary circumstances.’”
Kacsmaryk, however, could also call for a hearing, or ask for additional responses.
The defendants in the case – the FDA and Danco, which makes mifepristone – argued in separate briefs to the court that a decision against the drug’s approval would be unprecedented and would shutter the drugmaker’s business.
Reproductive rights advocates have stressed that a ruling in favor of the plaintiffs would be devastating, with NARAL Pro-Choice America saying in a statement that if the drug is yanked from the market, “64.5 million women of reproductive age in the US would lose access to medication abortion care, an exponential increase in harm overnight.”
CNN’s Tierney Sneed contributed to this report.