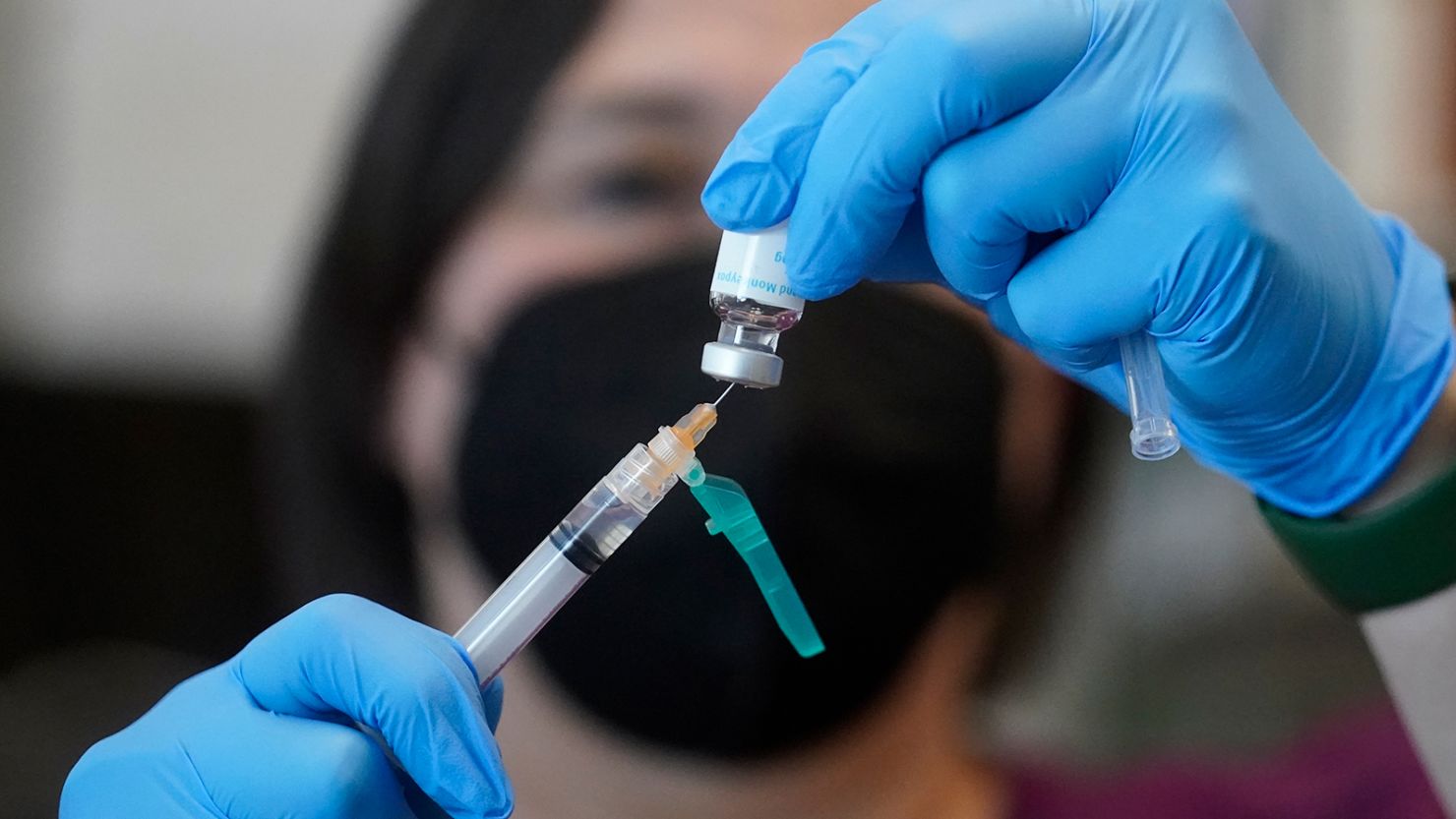
The US Food and Drug Administration defended the federal government’s recent decision to stretch out its limited supply of the Jynneos monkeypox vaccine by giving individuals smaller doses using a different method of injection, pointing out in a letter to the company that manufactures the vaccine that a 2015 clinical study showed “a very similar immune response” to the new method as well as the previously used one.
In a letter Friday to Bavarian Nordic CEO Paul Chaplin, two top FDA officials responded to concerns Chaplin had raised earlier this week. They wrote that the agency recently determined that the benefits of stretching its limited supply of the two-dose Jynneos vaccine by giving individuals smaller doses outweighed the known possible risks.
The decision came after the FDA ruled out – at least for now – the use of alternative vaccines, as well as the option of delaying the second vaccine dose by three to six months, the agency said.
The FDA’s letter, obtained by CNN Saturday, was signed by FDA Commissioner Dr. Robert Califf and Dr. Peter Marks, the director of the FDA’s Center for Biologics and Evaluation Research.
The letter cited a 2015 clinical study, which Chaplin co-authored, in which “individuals who received the vaccine intradermally received a lower volume (one fifth) than individuals who received the vaccine subcutaneously.”
“The results of this study demonstrated that intradermal administration produced a very similar immune response to subcutaneous (SC) administration,” the letter said. While the intradermal injection method “did result in more redness, firmness, itchiness and swelling at the injection site, but less pain,” the letter continued, “these side effects were manageable.”
The FDA also detailed other options that the agency had considered. But the use of alternative vaccines to prevent monkeypox “was determined to be either impractical or inadvisable at this time,” according to the letter. Specifically, the ACAM2000 vaccine, which is FDA approved for prevention of smallpox, “may not be appropriate now for a potentially immunocompromised population,” the letter said.
The FDA also looked into the option of delaying the second vaccine doses by three to six months, rather than giving the second dose the recommended 28 days after the first.
But the agency ultimately determined that there was not any data to demonstrate that this method would provide sufficient protection, and that delaying the second dose could also give people “a false sense of reassurance that they were protected against monkeypox when the actual level of protection would be unknown and quite possibly inadequate,” the letter said.
The federal government’s announcement this week authorizing health care providers to give smaller doses of Jynneos came in an attempt to better meet the high demand for the vaccine. Top Biden administration health officials have made clear in recent days that given the speed of the spread of monkeypox – which it earlier this month declared a public health emergency – the US did not have enough vaccine supply to meet demand. This week’s decision to stretch out the available Jynneos vaccines was aimed at addressing the limited supply.
Dr. Marks of the FDA said Thursday that there were an estimated 1.6 million to 1.7 million people in the US who are now eligible for the two-dose Jynneos vaccine. According to the US Department of Health and Human Services, around 634,213 vials have been sent to jurisdictions as of Friday.
The low-dose intradermal strategy immediately prompted concern among some public health experts, including about the limited amount of research surrounding the new method.
“This approach raises red flag after red flag, and appears to be rushed ahead without data on efficacy, safety, or alternative dosing strategies,” David Harvey, executive director of the National Coalition of STD Directors, said in a statement this week.
Chaplin, the CEO of Bavarian Nordic, also raised concerns. CNN reported that Chaplin wrote in a letter Tuesday to Califf and HHS Secretary Xavier Becerra that he had concerns about the “very limited safety data available” on the newly announced vaccination strategy, and that a relatively high percentage of people in the clinical study – 20% – did not get their second shot.
“While we have certain reservations, we are trying to find the best way to support the [emergency use authorization] by collecting additional data and aligning on the responses to assist state officials in the rollout,” Chaplin wrote. “We are also investing in expanding the manufacturing capacity at both BN and external facilities, with likely more announcements soon to come.”