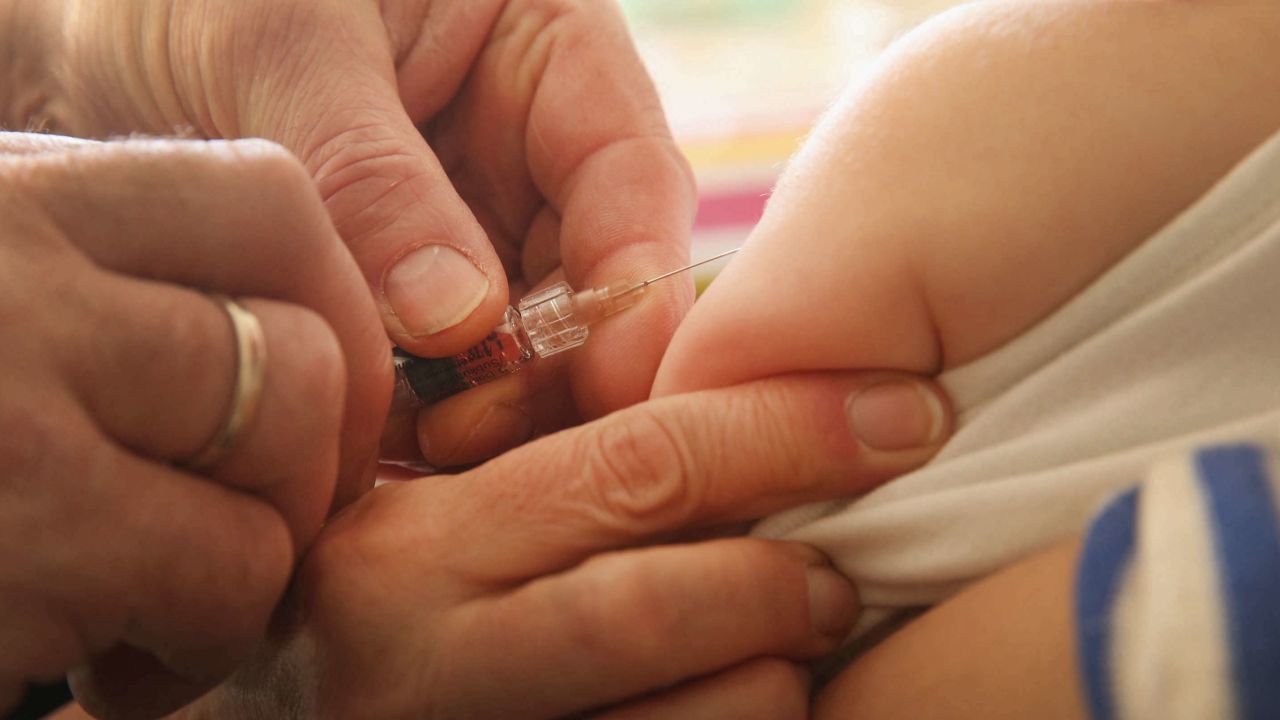
The timeline for when children younger than 5 in the United States might start receiving Covid-19 vaccinations has just been pushed back.
The US Food and Drug Administration announced Friday that it had postponed a meeting of its Vaccines and Related Biological Products Advisory Committee, which was intended to review data on Pfizer and BioNTech’s request to authorize a two-dose series of their child-sized vaccine for children 6 months through 4 years old.
Instead, the agency is waiting for the vaccine makers to submit data from an ongoing trial on a three-dose regimen in these younger children before moving forward with consideration of an emergency use authorization, allowing the FDA to review all data available on the efficacy of each regimen option.
Pfizer and BioNTech said Friday that they expect to have data on three doses available in early April.
The Pfizer/BioNTech vaccine is authorized for use in people as young as 5. If the new EUA is granted, this shot will be the first coronavirus vaccine available for the youngest children – and the tentative plan is to roll out about 10 million vaccine doses initially, according to a US Centers for Disease Control and Prevention document.
Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia and a member of the FDA vaccine committee, said he’s actually glad things played out this way.
If the committee had met as planned, on February 15, and voted against authorizing the vaccine based on the two-dose data, “that would have sent the message to the general public that something’s wrong with this vaccine. And then [if] we come back a few months later and vote yes, people may still be suspicious or less likely to take the vaccine up,” Offit said.
“My word to parents who have young children is that this is not a ‘no’; it’s a ‘not yet,’ ” he added.
What needs to happen before shots can roll out?
Before Pfizer’s coronavirus vaccine can be administered to children younger than 5, the FDA needs to authorize it for emergency use in that age group.
Once the FDA vaccine committee meeting is back on, it will review the data from clinical trials in Pfizer/BioNTech’s EUA request and recommend to the FDA whether it supports authorizing the vaccine, and then the FDA will decide whether to grant emergency authorization.
If the FDA authorizes the vaccine for young children, the CDC’s vaccine advisers – the Advisory Committee on Immunization Practices – will meet to discuss the EUA, review data on the vaccine and vote on advising the agency to recommend using the vaccine.
“The FDA uses data submitted by the manufacturer. ACIP can use all kinds of other data to consider in their deliberations,” Dr. Sean O’Leary, vice-chair of the American Academy of Pediatrics Committee on Infectious Diseases, told CNN.
Next, CDC Director Dr. Rochelle Walensky reviews ACIP’s vote and could recommend the vaccine for children under 5, giving the green light for vaccines to be administered to that age group.
“At that point, then, it just depends on getting the vaccine shipped into places that they’re going to be delivering the vaccine,” said O’Leary, who is also a professor of pediatric infectious disease at the University of Colorado School of Medicine and works with Children’s Hospital Colorado.
How soon can young kids get vaccinated?
Pfizer and BioNTech expect to have data on three doses available in early April, and the FDA said it will move quickly to review the data and consider an emergency use authorization.
Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said Friday that he understands that parents are eager to get protection for their young children, and the agency is “absolutely committed to moving as rapidly as we can once we have a submission.”
In December, Pfizer announced that it decided to add a third dose to the primary vaccine regimen for babies and children ages 6 months to up to 5 years. That decision came after initial trials in children ages 2 to 5 showed that the original two-dose regimen of the child-sized vaccine did not provide the expected immunity in the 2- to 5-year-olds, although it did so for the babies up to age 2.
The companies have said that data on a third dose given at least eight weeks after the second dose is expected in the coming months and will be submitted to the FDA.
There are about 18 million children ages 6 months through 4 years in the United States who potentially will be eligible to get vaccinated against Covid-19.
Operationally, the United States will be ready to roll out the vaccine for children under 5 once the FDA and CDC make their recommendations, Jeff Zients, the White House’s coronavirus response coordinator, said at a news briefing earlier this month.
“We can start packing and shipping the vaccine once FDA makes its decision,” he said.
“We’re working closely with pediatricians and family doctors and children’s hospitals and pharmacies to make sure the vaccine is available at thousands of locations across the country.”
Pediatricians’ offices are expected to be among the main sites where children younger than 5 will be able to receive their Covid-19 vaccinations, according to O’Leary.
He added that public health departments and pharmacies could be some other locations.
“It gets a little trickier under 5 because a lot of pharmacies may not be accustomed to giving vaccines to children under 5, so that will depend on the various pharmacies,” he said. “We know that a lot of the current vaccination is happening in pharmacies, but more and more are happening in primary care offices, where pediatricians and family medicine physicians are very used to giving vaccines to younger kids.”
O’Leary added that many of the pediatricians’ offices that are now administering Covid-19 vaccinations to children ages 5 to 11 are likely to also be sites for vaccinations for younger children.
The vaccine will be administered as an injection in themuscle, given as two doses, about three weeks apart.
Why was the EUA request originally delayed?
Pfizer’s decision to extend its vaccine trial in younger children and test a three-dose regimen delayed its initial application to the FDA for authorization of its vaccine for children younger than 5.
The company decided to add the third dose – a 3-microgram shot given at least two months after the second dose – for all children and babies ages 6 months to 5 years after its independent outside advisers, the Data and Safety Monitoring Board, viewed the data, which showed the vaccine doses were not providing the protection from infection expected among 2- to 5-year-olds.
There were no safety concerns.
“Previously, we had data showing that the childhood vaccine for 6 months to 4 years wasn’t as protective against infection as the adult vaccine. That’s the reason why they pushed it out and asked for that third dose,” former FDA Commissioner Dr. Scott Gottlieb said on CBS’s Face The Nation this month.
“But now, if the goal of the vaccine is to get baseline immunity in the kids – to prevent really bad outcomes – and you’re really not using the vaccine as a tool to prevent infection in the first place, two doses could do that,” Gottlieb said. “I think that may be why federal health officials are rethinking this.”
How is the vaccine for younger children different from the others?
For children younger than 5, Pfizer and BioNTech already had reduced the vaccine dosage size. For the 12-and-up age group, the dosage has been 30 micrograms of vaccine. Pfizer and BioNTech stepped that down to 10 micrograms for children 5 to 11, and took it even lower – to 3 micrograms per dose – for children younger than 5.
“In the under-5, the dose that they landed on – based on looking at different doses in earlier trials – was 3 micrograms, so one-tenth of what we saw in what we’re using in the adults,” O’Leary said.
Early tests had indicated that this 3-microgram dose would produce a strong immune response in the children and minimize the risk of side effects.
What do we know about parents’ willingness to get kids vaccinated?
Once vaccine doses are authorized for children younger than 5, 31% of parents of children in this age range say they’ll get their child vaccinated right away, up from 20% in July, according to survey results from the Kaiser Family Foundation.
The survey of more than 1,500 adults, conducted in January, found that another 29% say they will “wait and see” before getting their child under 5 vaccinated, down from 40% in July. About 1 in 10 parents, or 12%, say they’ll vaccinate their child under 5 “only if required,” while about a quarter – 26% – say they will “definitely not” vaccinate their young child.
Get CNN Health's weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
Until younger children are eligible to get vaccinated, “we’ve got to do our best to protect them,” Dr. Stephen Parodi, national infectious disease leader for Kaiser Permanente, told CNN in November.
“For the youngest children that we have, we still got to take those protective measures when it comes to distancing, and ideally, if people are coming into the household, that they have gotten vaccinated so that you’re minimizing the risk,” Parodi said, adding that mask-wearing is key too.
CNN’s Jen Christensen and Brenda Goodman contributed to this report.