(CNN)After a day of celebration and heartache, Americans face a harsh reality with the Covid-19 crisis.
A record 112,816 Covid-19 patients were hospitalized Tuesday, according to the Covid Tracking Project.
That will inevitably lead to more deaths as Christmas and New Year's Day get closer.
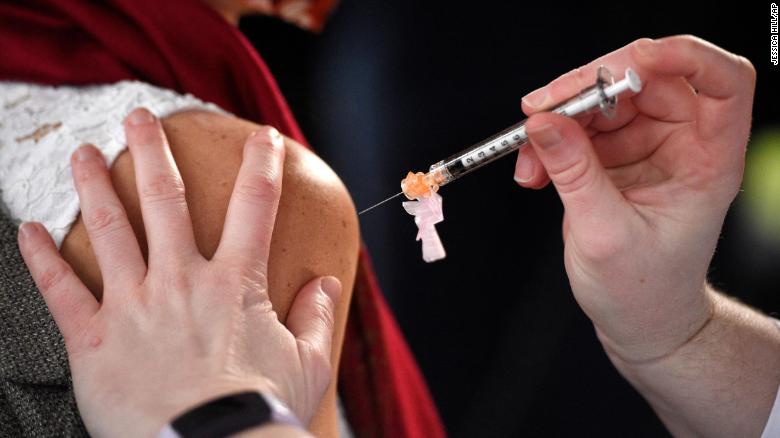
And while more doses of the Pfizer/BioNTech vaccine are sent across the country this week, there won't be enough for everyone who wants it for months.
"This vaccine, as wonderful as it is, is not going to change the trajectory of what we experience this winter," said Dr. Richard Besser, former acting director of the US Centers for Disease Control and Prevention.
"It's not going to change what we need to do. It's not going change the need for us all to wear masks, and social distance and wash our hands."
The FDA authorizes a fully at-home test
The US Food and Drug Administration gave emergency authorization Tuesday for the first Covid-19 test that can be fully taken at home.
Other at-home tests require a prescription or require people to send test samples to a lab to get results. But the Covid-19 home test developed by Australian company Ellume is sold over-the-counter and produces results that can be read at home.
"Today's authorization is a major milestone in diagnostic testing for COVID-19," FDA Commissioner Dr. Stephen Hahn said in a written statement. "By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results in as little as 20 minutes."
The test uses an analyzer that connects with a software application on a smartphone to help users perform the test and interpret results, the FDA said.
The Ellume test is an antigen test that "correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms," the FDA said.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
Testing Americans is an important issue, Dr. Sanjay Gupta, CNN chief medical correspondent, said.
"This is one of the untold or under-told sort of issues of this entire (pandemic)," he said. "I think it would be a big deal if they could produce enough of these tests to get them in most, if not all, households in America."
Being tested on a regular basis with rapid results could help inform people when to stay home or go out, he said.
"That type of testing is critically important, has been critically important and will remain critically important," he added.
Because the test isn't perfect, people who get a negative result should still presume they may be infected and act accordingly -- wearing a mask and keeping away from others.
Ellume expects to produce more than 3 million tests in January. When it applied for emergency use authorization, the company said it would charge $30 for the test.
Shutdown decisions on the minds of officials
As doctors and nurses across the nation roll up their sleeves to get vaccinated, governors and mayors are having to consider implementing stricter health measures to blunt the impact of the virus before inoculations become widespread.
New York Mayor Bill de Blasio told CNN on Tuesday that while it's up to the state's governor, he would recommend tight stay-at-home orders for the holidays.
"We've seen the numbers go up. We are concerned about our hospitals, protecting the ability of hospitals to serve people. Clearly we are going to need a pause, we're going to need a shutdown," he said. "And I think the sooner the better."
The mayor has said after Christmas might be a good time for a shutdown but on Tuesday, de Blasio said if Gov. Andrew Cuomo wants to shutdown sooner, he would agree with that.
De Blasio said a shutdown should last between two and four weeks, because that's the amount of time it takes before the effects of a shutdown can be measured.
Many people are worried about the financial impacts of mandates. This week, the city shut down indoor dining for two weeks, something that is going to affect the bottom line of restaurants.
"We have pivoted, we've changed, we've opened, we've done everything that has been asked," said Melba Wilson, owner of Melba's Restaurant in Harlem and the president of the New York City Hospitality Alliance. "I don't know that we can come back from this," she added.
California is in the middle of stay-at-home orders in the most populated regions of the state based on ICU capacity. Cases, deaths and hospitalizations continue to rise and the number of ICU beds available continues to fall.
Boston just moved to more restrictive measures on public activities, limiting gathering size. Indoor dining is limited to 90 minutes, according to Mayor Marty Walsh.
"I want to be clear, this is not about targeting specific sectors, this is an effort to reduce overall activity outside the home, he said. If the upward trend doesn't slow or reverse, it will be a very difficult winter, he told reporters.
What's next with vaccine distribution
About 2.9 million doses of the Pfizer/BioNTech vaccine either have been or will be received nationwide this week. Another 2.9 million doses are being held back because they are required in 21 days for the second dose, officials have said.
In all, about 20 million people should get their first shots this month.
By the end of this week, the FDA could give emergency use authorization to another vaccine -- this one made by Moderna.
If authorized, the US plans to start by shipping about 6 million doses across the country, said Gen. Gustave Perna, chief operating officer of Operation Warp Speed.
Just like the Pfizer/BioNTech vaccine, the Moderna vaccine requires two doses. It's up to states to allocate their share of vaccines.
The CDC has recommended that health care workers and residents of long-term care facilities get the vaccine first.
But vaccines will have little impact on what's already happening: a devastating season that just saw record-high deaths, hospitalizations and new cases.
In the past week, the US has reported an average of more than 215,000 new infections a day, according to Johns Hopkins University.
Infections have increased significantly since Thanksgiving gatherings, and officials say upcoming holiday gatherings will add fuel to the fire.
More than 303,000 people in the US have died from coronavirus in 10 months, according to Johns Hopkins.
And another 186,000 are projected to die over the next three months, according to the University of Washington's Institute for Health Metrics and Evaluation.
Overcoming vaccine hesitancy
To help the US get to the other side of this crisis, health officials are trying to tackle skepticism about the vaccine.
"Nothing has been in my heart more than this issue over the past several weeks to months," US Surgeon General Dr. Jerome Adams said.
"I've been working with Pfizer, with Moderna, with AstraZeneca, with Johnson & Johnson to make sure we have appropriate numbers of minorities enrolled in these vaccine trials so that people can understand that they are safe."
"There are tens of thousands of Black and brown people dying every year because they are distrustful of the system," Adams said. "In many cases, rightly so, but also because they're not getting the facts to help restore their trust in the system."
Sandra Lindsay, an intensive care unit nurse at Long Island Jewish Medical Center in Queens, New York City, was among the first Americans to get a shot of the vaccine.
"I understand the mistrust among the minority community," said Lindsay, who is Black. "I don't ask people to do anything that I would not do myself. And so I was happy to volunteer to be among the first."
She said she didn't know she'd make history as one of the first members of the public to get a Covid-19 vaccine.
"That's not why I did it," Lindsay said. "I wanted to do it to inspire people who may be skeptical about taking the vaccine and trust in the science."