Story highlights
Volunteers are crucial to the success of Zika vaccine trials
"It's clearly altruism" that drives most volunteers, says study director
It’s another life-defining moment for 33-year-old Virginia Bliss. She’s about to receive one of the first doses of an experimental Zika vaccine administered at the Hope Clinic in Atlanta, part of the clinical trials unit of the Emory Vaccine Center. She’s one of an army of volunteers the clinic relies upon to test new vaccines.
“We really lean on our vaccine soldiers,” said Hope’s medical director, Dr. Sri Edupuganti. “That’s why the first few people we bring in for a new study are already familiar with our routine and what is expected of them.”
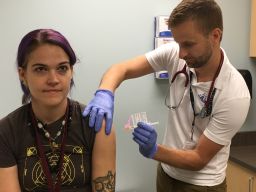
“I like that,” Bliss responded with a laugh. “Vaccine soldiers – I don’t mind being part of that army!”
Bliss is one of 80 healthy men and women, ages 18 to 35, who are testing the safety of a DNA-based Zika vaccine developed by the National Institute of Allergy and Infectious Diseases. The first injection occurred in early August in Bethesda, Maryland; the other study sites are in Baltimore and Atlanta.
Related: Human trials begin for Zika vaccine
Animal models show encouraging results. The vaccine has produced “really good, strong antibodies” against Zika in mice, said Edupuganti, and a just-released study showed that 17 of 18 rhesus macaque monkeys were protected from Zika after two doses of the vaccine.
The government hopes to have safety results from this human trial by the end of year. If all goes well, Edupuganti said, the vaccine will enter phase 2 trials in early 2017, targeting areas such as Puerto Rico and Central America, where Zika infections are widespread.
Becoming a vaccine soldier
Most of us might shudder at the idea of allowing an experimental vaccine into our bodies, but Bliss is a vaccine veteran. Over the past eight years, she’s volunteered to test vaccines for yellow fever, Ebola and meningococcal disease. Money is definitively not a motivation; volunteers are paid only about $20 an hour for their time.

“I really believe in medical research,” Bliss said as she offered her arm to a nurse for a blood draw. “I’m just glad I can contribute to medical history.”
But Zika? Yellow fever? Ebola?
“A lot of my friends and family were terrified,” Bliss admitted with a smile. “But I’m very comfortable with it. What people don’t realize is that the vaccines they are using on humans have already been through so many rounds of animal testing, by the time it gets to humans, it’s been pretty well vetted.”
It helps that Bliss understands how vaccines are developed. She has a background in biological science and works as a histologist at Atlanta’s Yerkes National Primate Research Center, which conducts biomedical and behavioral studies with nonhuman primates.
But for this study, her motivation was more personal.
“I’m a mother, and I can only imagine how devastated I would have been if my child had a very serious birth defect because of an illness,” she said. “So to be able to save other mothers from this potential heartache would be wonderful.”
The race for a Zika vaccine
“We need a vaccine for this epidemic as soon as possible; there is no question about that,” Edupuganti said. “We’ve all seen the pictures of the newborns born to women infected with Zika, babies born with small heads and brains. That’s devastating.”
Zika is entrenched in over 60 countries around the world, with Puerto Rico, the Caribbean, and Central and South America especially hard-hit. Thousands of unborn babies are at risk for birth defects, such as microcephaly, in which the head and brain stop developing, as well as vision and hearing defects and learning disabilities.
Another reason to fear Zika: It can trigger Guillain-Barré, a disorder in which the body’s immune system attacks nerves, leading to potential paralysis and even death.
Scientists around the world are scrambling to create viable versions of a Zika vaccine. One of the most advanced efforts belongs to Inovio Pharmaceuticals and GeneOne Life Science, which are testing the safety of a synthetic DNA vaccine on 160 people in Puerto Rico.
The government vaccine Bliss is receiving is also DNA-based, which the agency says is safer than a traditional vaccine that includes a whole killed virus, because it does not contain infectious material.
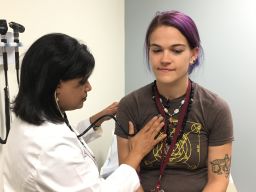
“There is no Zika virus in this vaccine whatsoever,” Edupuganti explained. “This particular vaccine encodes two proteins of Zika. Once it’s injected into the body, the cellular mechanism in our bodies will read that genetic code and translate it into the proteins of the Zika virus and then react to it.”
Edupuganti believes the vaccine is especially impressive due to the speed with which it has been developed. That’s because it is based on a DNA-based West Nile vaccine developed by the government that never made it to market.
“Simply put, they had the platform to remove the West Nile proteins from the vaccine and insert the Zika,” Edupaganti said, “which is why it’s only taken eight months or so to develop this option, rather than the years and years it would take to start from ground zero.”
But even with this huge head start, vaccine development takes time. The World Health Organization has said not to expect any sort of Zika vaccine to hit the market before mid-2018.
A huge commitment
The first day on the job as a Zika “vaccine soldier” is time-consuming. Bliss must go through hours of paperwork and a full physical exam and give what seems to be copious amounts of blood. Half will be used to test existing immunities; the other will be frozen for future comparisons.
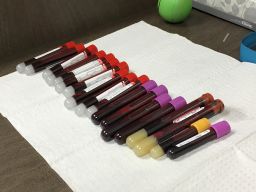
“I think I’ve given more in the past,” she said calmly as the nurse started yet another vial. It turns out that the 18 vials, color-coded for identification, are less than what one would donate at a Red Cross blood drive.
A computer randomly assigns her into one of four groups, determining how many vaccinations she will get over a 20-week period. Though the rhesus monkey study showed that two doses are necessary to develop immunity, that has yet to be proved in people.
But suffering through a shot is just the beginning of her responsibility. After each injection, Bliss must monitor her temperature and any symptoms such as nausea, chills or aches and pains as well as injection-site reaction for seven days in a journal.
Most of the time, there’s not much to report. But during the Ebola vaccine trial, she developed flu-like symptoms, a reaction to the viral host that was used to contain the Ebola DNA.
Bliss said she told her family and friends that the vector used in the Ebola study was probably a cold virus. “They said, ‘Well, how do you know? You’re being injected with these experimental substances!’ And I said ‘it’s just the protein; you can’t get Ebola from the protein,’ ” Bliss said with a shrug.
For the Zika vaccine trial, Bliss is expected to come back to the clinic for checkups on a regular basis, once a week or so, with longer follow-up exams at 18 and 24 months.
All in all, it’s a two-year commitment that is crucial to the study’s success.
“It’s critical that a volunteer finish all of the visits in the study,” Edupuganti said. “That two-year time point is measuring how robust the immune response will be. So if someone was to drop out in the middle, we would not be able to gather all of the data that the study was designed to discover.”
“It’s a pretty big time commitment,” Bliss said, “but I feel being a part of these studies has enriched my life. I have talked to my daughter quite a bit actually about the vaccine studies that I do and about our obligation as educated people to always be working toward a better future. “
Follow CNN Health on Facebook and Twitter
And if you think Bliss’s dedication is unusual, think again. Edupuganti says the Hope Clinic received an enormous response to its call for volunteers.
“As soon as we announced the study, our recruiter couldn’t keep up with the calls,” she said. “And that’s why it’s exciting to come to work here every day. You have all of these people coming in to help, but they don’t have to be here; they are volunteers. So you better get up and get to work!”