As a triple threat of respiratory illnesses – flu, Covid-19 and respiratory syncytial virus, known as RSV – sweeps the United States, emergency departments are using one small tool more than usual to monitor whether a patient needs oxygen: the pulse oximeter.
“We’re in the midst of a respiratory flood,” said pediatric emergency physician Dr. Joseph Wright, chief health equity officer at the University of Maryland Medical System, which includes 11 hospitals.
“And the pulse oximeter is used from any age to geriatrics,” he said. “This is a tool that is used on all patients, and right now, as with the height of the pandemic, it’s a tool that is used to assess children with respiratory distress as part of the RSV flood that we’re currently experiencing.”
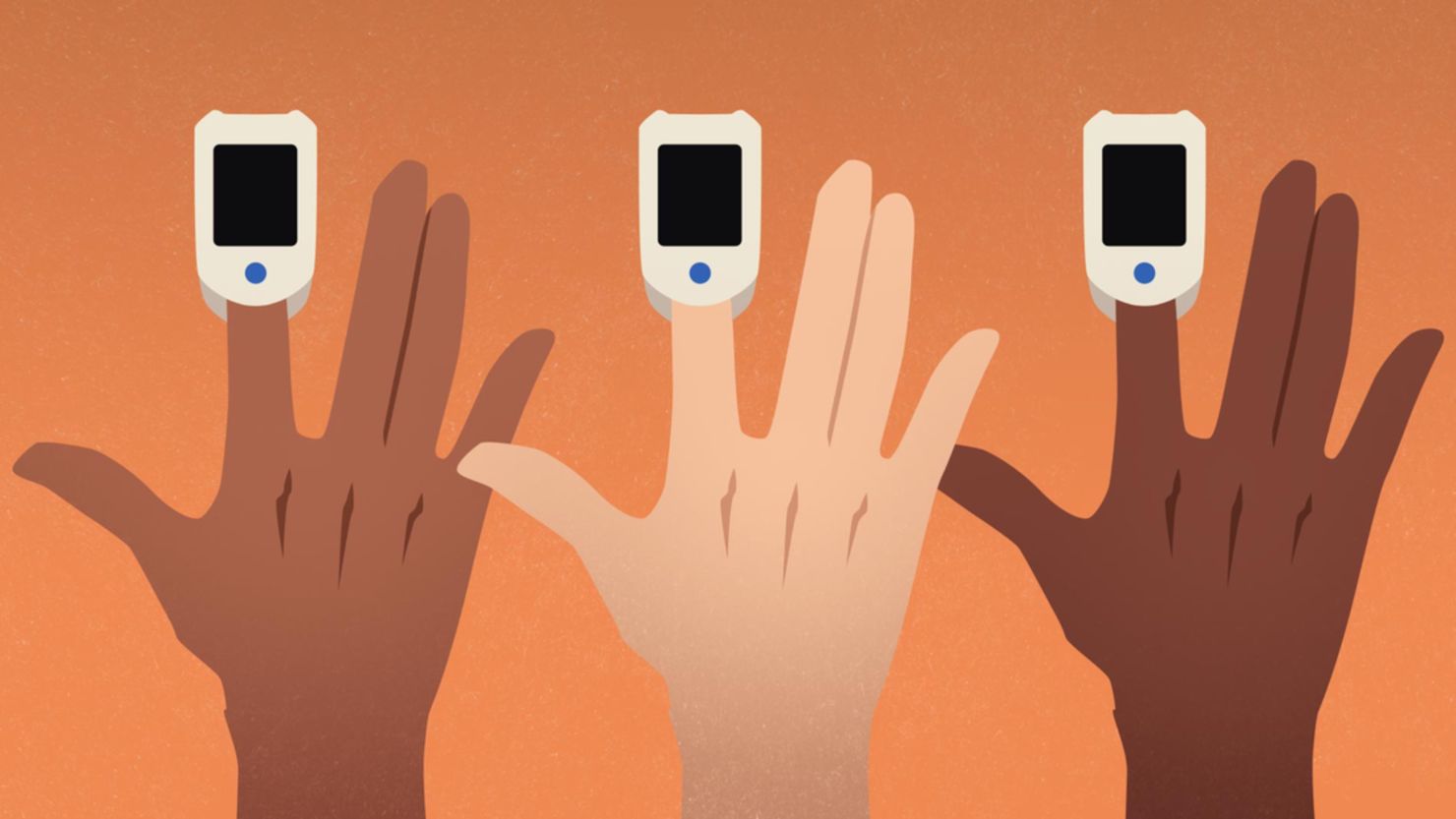
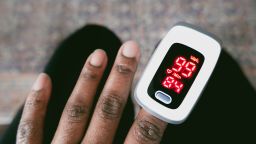
But a growing body of research suggests that these devices, which clamp onto a patient’s fingertip to measure their blood oxygen levels, may not work as well on people with dark skin tones.
The US Food and Drug Administration is mulling over next steps for the regulation of pulse oximeter devices, which may give less accurate readings for people of color. A panel of its Medical Devices Advisory Committee met in November to review clinical data on the issue.
“For all of us, we would like to have assurance or confidence that the accuracy of the pulse ox reading in children who are melanated or have darker skin tones is reliable,” Wright said. He was not involved in the FDA discussions, but his medical system offered written testimony for the meeting.
“When I’m assessing a patient, a child, who is in respiratory distress, the pulse ox reading is but one tool. There’s the clinical assessment, obviously, and then other measures of how sick that child is,” he said, but “these devices need to be fixed. It appears that the technology to fix them is known, and the advancement here is to require manufacturers to incorporate this advanced technology.”
FDA considers how to move forward
Pulse oximeters work by sending light through your finger; a sensor on the other side of the device receives this light and uses it to detect the color of your blood. Bright red blood is highly oxygenated, but blue or purplish blood is less so.
If the device isn’t calibrated for darker skin tones, melanin – which is responsible for the pigmentation of skin, hair and eyes – could affect how the light is absorbed by the sensor, leading to flawed oxygen readings.
The members of the FDA advisory panel discussed recommendations on when and how to use these devices on people with dark skin, how to improve their accuracy and, until the situation improves, whether the devices should have labels – such as a black box warning, the strongest type of warning for medical device or prescription drug labeling – noting that inaccurate readings may be associated with skin color.
“The agency considers this a high priority and we will work expeditiously to consider the Panel’s input and determine the appropriate next steps,” FDA spokesperson Shauna Nelson wrote in an email to CNN. “We will communicate any significant new information publicly.”
Meanwhile, the American Medical Association adopted a policy last month calling for the FDA to ensure that pulse oximeters provide accurate and reliable readings for people of all skin colors.
“Concerns about the accuracy of pulse oximeters in pigmented skin have been noted for more than 30 years, yet Black and Brown communities are still facing adverse health impacts from these devices – particularly during the COVID-19 pandemic when use of and reliance on pulse oximeters increased,” AMA President-elect Dr. Jesse Ehrenfeld said in a statement.
“We urge the FDA to take swift action to address the growing uncertainty around these devices, including making sure health care professionals are aware of their limitations and increase testing of devices that were already cleared by the agency, to ensure the health and safety of the public.”
‘We are many shades of brown’
Rekha Hagen told the FDA advisory panel during its meeting that she has seen a pulse oximeter give different readings for various members of her family, based on their skin tones.
Speaking as a member of the patient and family advisory council at the Hospital of the University of Pennsylvania, Hagen said that she is an Indian woman, her skin tone differs from her husband’s, who is White, and from those of their three children.
“In other words, we are many shades of brown and white,” she said.
“It’s very important to have an accurate reading because people are acting, or not acting, on this information. For example, if your thermometer says you have a temp of 105, you would treat it differently from a temperature of 101,” Hagen said. “I think of the pulse oximeter reading in the same way. And frankly, if the reading was acceptable, I would not go to the hospital or seek help. Of course this can be dangerous.”
Ultimately, the pulse oximeter can estimate the amount of oxygen a person has in their blood without the need for a blood sample.
But on a person with darker skin, the oximeter could indicate that oxygen levels are normal, suggesting that the person may be discharged from a hospital or may not need oxygen support – when a blood sample might show that, in fact, their oxygen levels are low, suggesting that they need additional care and oxygen.
Hagen asked the panel, “Since we have many skin tones in our immediate family, who would we use this device on?
“As for current solutions for the FDA, perhaps you could have a skin tone color chart on the box whereby you are advised not to use the product if you are darker than a certain skin tone or sell the oximeter behind the pharmacy counter so that the pharmacist can explain usage to the patient,” she said. “The FDA has time to fix this communication. They should start now.”
In order to resolve the core issue of flawed pulse oximeter readings, the FDA must expand premarket testing of the devices to include people with a broad array of skin colors, Dr. Ealena Callender of the National Center for Health Research said during the meeting.
The FDA now recommends that every clinical study of pulse oximeters include participants who vary in age and gender, with a range of skin pigmentation, of which at least two people or 15% of the group – “whichever is larger,” the FDA guidance indicates – have dark skin.
“This is woefully inadequate,” Callender said.
She added that “dark skin” tends to be subjective, and there is a need for objective tools to make that call.
“Only objective tools for assessment of skin pigmentation should be used in studies of how it affects pulse oximetry measurements,” Callender said, explaining that many variations in hue and other contributing factors make subjective assessments less accurate.
“In general, inaccuracies related to skin pigmentation increase as the level of oxygenation decreases. Clinically, this means sicker patients are less likely to get an accurate reading, and are therefore less likely to get appropriate care,” she said. “The FDA should require more scrutiny to minimize bias in medical devices so they are accurate and reliable for everyone.”
The FDA panel discussed certain skin color charts, descriptors and scales that have been used in medicine to determine a person’s skin tone, but those too can be subjective. None of those scales indicates how much melanin a person has in their skin.
There are technologies, such as spectrophotometry, that can measure how much a chemical substance absorbs light and provide an objective measurement of melanin in the skin, but such spectrophotometers in the lab can cost thousands of dollars.
All pulse oximeters need to be calibrated in humans in order for the optical signals used in the device to translate and produce an accurate oxygen saturation reading, Dr. Philip Bickler, professor and director of hypoxia research laboratory at the University of California, San Francisco, who has been studying pulse oximeters, said during the FDA panel meeting. Researchers at UCSF are working on a project called the Open Oximetry Project to improve equity in oximetry.
“You can imagine that if all the calibration procedures are done in subjects with low skin melanin, you produce one marker that would produce pulse oximeters that would be accurate in individuals with lightly pigmented skin – and what has become apparent is that it’s been insufficient to account for the presence of melanin,” he said.
“Now, you could do another calibration for subjects with darkly pigmented skin and you would get a different calibration curve,” he said. “So that is possible – and almost 20 years ago, we advocated for something like that.”
Pulse oximeters were invented in 1974, and a body of research – dating to the 1980s – suggests that flawed pulse oximeter readings among Black and brown patients can be a real and life-threatening issue in medical care.
‘A prime example of valuing Black lives less’
This difference in how pulse oximeters perform for people with dark skin tones compared with those who have fair skin can drive racial disparities in the care patients receive.
“This is distinct from some of the other race-based inequities that we’re currently tackling in health care. This one is really clear. It’s very straightforward what the scientific solution is,” the University of Maryland Medical System’s Wright said. “Here is an example where we have a very clearly defined biologic reason for why the infrared wavelengths of light don’t penetrate to detect oxygenation in folks with melanin as opposed to those without.”
Another distinction: There has been evidence of colorism, or prejudices or discrimination against people with darker skin tones, playing a role in racial biases and the medical care some people get. Historically in medicine, medical data has involved a person’s race and not their skin color. Yet there are both light-skinned and dark-skinned Black people, Asians, Pacific Islanders, Native Americans and Hispanic people, and within each of those racial and ethnic groups, skin tone could play a role in biases in medical care.
But the focus on specific skin tones – not race – when addressing the risk of inaccurate pulse oximeter readings appears to be “rooted in a very real desire to avoid medicine’s long and deeply appalling history” of disparities that arise when Black and brown communities are not provided the same quality of care as White populations, said Dr. Theodore J. Iwashyna, professor of pulmonary and critical care medicine, and of health policy and management, at Johns Hopkins University.
The greater error rate in pulse oximeters for people with dark skin “is a prime example of valuing Black lives less,” said Iwashyna, who has studied how racially biased oxygen readings could put patients at risk.
“There is a potential profound crisis that paying attention to these racial differences has made visible, in a ubiquitous device, that is disproportionately hurting Black patients,” he said. “And if attending to that difference can yield a set of monitoring devices that allow us to more safely and effectively care for all patients, including Black patients, that seems great.”
In October, Iwashyna and two other researchers at the University of Michigan – Dr. Michael Sjoding and Dr. Thomas Valley – wrote an editorial, published in the American Journal of Respiratory and Critical Care Medicine, calling for the FDA to require pulse oximeter manufacturers to report how their devices perform in patients from diverse racial backgrounds. They wrote that the focus should remain on racial differences in accuracy until skin tone has been confirmed as “the underlying mechanism” for those discrepancies.
“There are clearly these differences by race. And I think, as you read the historical record over the last 30 years, the reason those differences in accuracy were tolerated for so long is not because of physiology but because of a social valuation as to which patients these devices were less accurate in, and whether that was considered an unacceptable error,” Iwashyna said.
At this point, he added, conversations should focus on fixing pulse oximetry inaccuracy in sick patients rather than the specific skin tones affected by the error.
“We could just fix the damn problem,” he said. “Let’s build devices that work better and are calibrated across our entire population. We know, from NASA’s work in the 1960s, that this is possible – just it has not been done.”
Pulse oximeter developers weigh in
In response to the discussion, the makers of some pulse oximeters have reported that their studies show no evidence of racial biases in the accuracy of their devices.
Studies of Medtronic’s Nellcor pulse oximeters found that they reported blood oxygen levels that were within 2% of participants’ drawn-blood oxygen levels – regardless of skin color, Dr. Sam Ajizian, chief medical officer of patient monitoring at Medtronic, said in an emailed statement to CNN.
“Still, the data shows a small statistical discrepancy between results for those with light pigmentation and patients with darker skin pigmentation,” Ajizian said.
“Medtronic is seeking to make improvements in our devices based on a greater understanding of the impact skin pigmentation has on pulse oximetry readings,” he said. “Through better information-sharing and an industry-wide commitment to continued innovation, we are advocating for improvements in the methods we use to validate pulse oximeters, including standardization of how we assess skin pigmentation and an increase in representation of patients with darker skin pigmentations in clinical trials.”
The medical technology company Masimo had similar sentiments.
“We have also calibrated and validated our oximeters using almost equal numbers of dark-skinned and light-skinned individual volunteers. We support prospective clinical studies, patient studies, on this topic, and we are pursuing these now,” Dr. William Wilson, Masimo’s chief medical officer, told the FDA advisory panel.
“Masimo supports raising the standard on requirements for the percentage of dark-skinned subjects used in calibration and validation studies,” he said. “We also believe it is important that the FDA regulates and applies similar oversight recommendations on all pulse oximeters, including those sold directly to consumers.”
Get CNN Health's weekly newsletter
- Sign up here to get The Results Are In with Dr. Sanjay Gupta every Friday from the CNN Health team.
Some experts worry that these studies of pulse oximeter devices in labs among healthy volunteers, as many manufacturers have done, might not be predictive of how the devices perform in medical centers among sick patients, indicating a need for more real-world data.
“The lab studies were really small,” Iwashyna said. “And maybe if the things worked for everybody, we wouldn’t have to spend forever trying to figure out which people they don’t work for, because they just work for everybody.”



![15943252: 3D Coronavirus Patients Lungs [Washington, D.C., U.S.]
A top hospital in Washington, D.C. has released the first images of a coronavirus patient's lungs, in an unprecedented 3D video.
The imagery shows extensive damage to an otherwise healthy, 59-year-old male who was asymptomatic until two to three days ago, said Keith Mortman, the chief of thoracic surgery at George Washington University Hospital. Now, as his lungs are failing, the patient requires a machine to help him breathe.](https://media.cnn.com/api/v1/images/stellar/prod/200325000535-covid-19-lung-damage-video.jpg?q=x_0,y_0,h_1125,w_1999,c_fill/h_144,w_256)