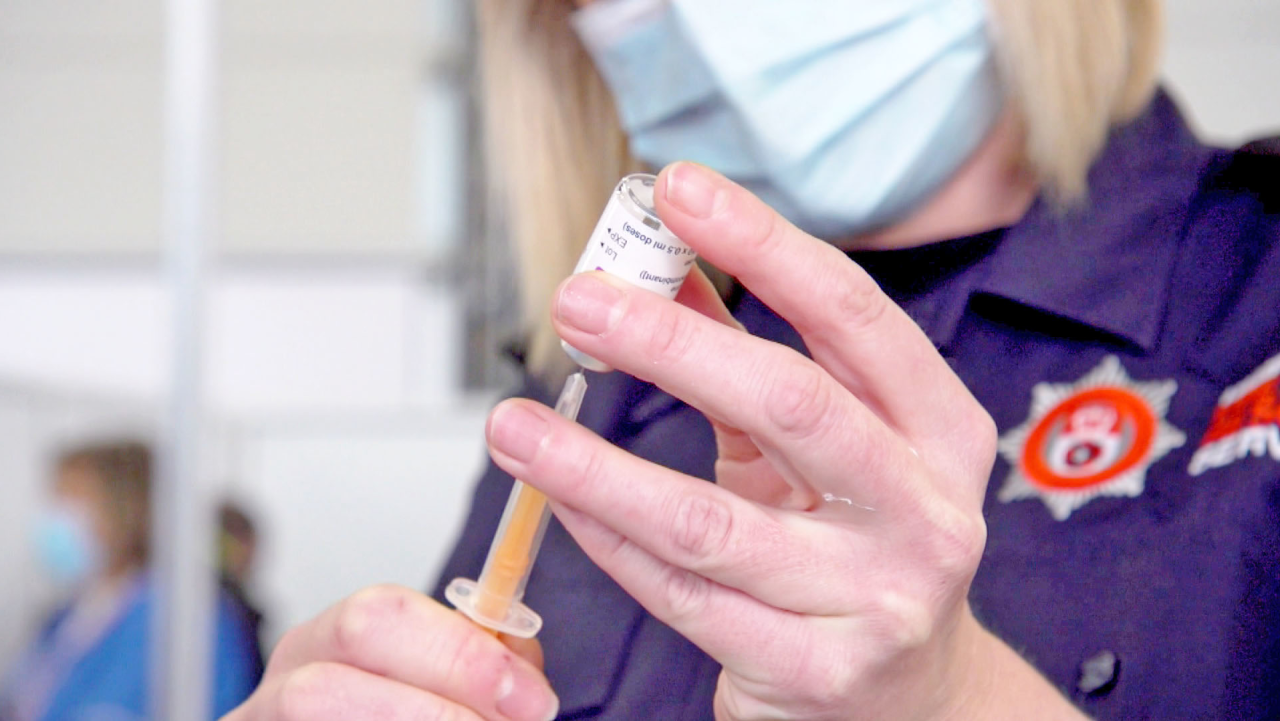
AstraZeneca’s contract to supply the UK with 100 million Covid-19 vaccine doses commits it to making “best reasonable efforts,” the same language used in its deal with the European Union, which critics blamed for the bloc’s faltering inoculation program.
The details of the contract are contained in a redacted version published online without fanfare months ago, long before the UK and the EU became embroiled in a bitter dispute over vaccine supply.
British officials had earlier declined to provide the contract to CNN, making no mention of the redacted version, and have repeatedly refused to give details on the country’s vaccine supplies, citing “security reasons.” A junior UK government minister said in a recent interview that publishing the contract would risk national security.
Yet in response to a Freedom of Information request from CNN, the Department for Business, Energy and Industrial Strategy (BEIS) this week provided CNN a link to the redacted 52-page contract, which had been published on a website that hosts details of UK government contracts. Details like the number of doses to be delivered to the UK and the dates of delivery have been redacted.
The redacted contract has, technically, been publicly available since at least November 26, according to the date the page was last edited. BEIS this week confirmed the same date of publication to CNN. But the link is difficult to find on the government website without using precise search terms and it appears to have gone largely unnoticed.
European Union leaders and AstraZeneca engaged in a public war of words in late January after the company advised the 27-country union that it would deliver tens of millions fewer doses than agreed by the end of March. At the same time, it appeared to be making good on its deliveries to the UK, heightening tensions between Westminster and Brussels, fresh from their Brexit divorce.
The EU then published its own redacted agreement with AstraZeneca. A comparison between the two contracts is now possible.
The UK is currently celebrating a hugely successful vaccination drive, having administered at least one vaccine dose in a two-dose regimen for more than 15 million people, over 20% of its population. The EU, on the other hand, continues to have major supply problems. It reports it has administered more than 20 million doses, though many people have received two shots, so it is unclear exactly how many individuals have received at least a single shot. Either way, it has given doses to just over 4% of its population at best.
AstraZeneca CEO Pascal Soriot told Italy’s La Repubblica at the time that its agreement with the EU was “not a contractual commitment. It’s a best effort,” referring to language used in their contract.
Its contract with the UK, however, also states that company only needs to make its “best reasonable efforts” to stick to the original agreed delivery schedule, which the company could “update and refine” when necessary. The agreement says the company must notify BEIS at least 30 days before each delivery with a “firm and final” schedule.
Where there may be a significant difference is in which markets the drug company is prioritizing. Soriot confirmed to La Repubblica that his company had agreed to supply the UK before other markets, saying it was “fair enough” because the UK had reached an agreement with AstraZeneca earlier than the EU. But the UK’s official contract is actually dated August 28, one day after the EU’s contract.
It was a reasoning ridiculed by European Commissioner for Health and Food Safety Stella Kyriakides, who had come under intense questioning over whether the EU’s “best efforts” contract was significantly weaker than the UK’s.
“We reject the logic of first come, first served,” she told reporters. “That may work at the neighborhood butcher’s but not in contracts and not in our advanced purchase agreements.”
The contract also confirmed the UK could receive vaccines manufactured in the EU, another point of contention between Brussels and AstraZeneca during their spat.
BEIS did not share even its redacted AstraZeneca contract with CNN when asked in late January, and it cited “security reasons” when declining to give information on vaccine supply levels. LBC Radio, which like many media outlets appeared to also be unaware the redacted contract had already been published, pressed junior prisons minister Lucy Frazer at the time as to why the government would not make the contract with AstraZeneca publicly available. She replied that doing so would pose a national security risk.
“It risks the security of the vaccine, which risks the security of the people,” she said.
AstraZeneca declined to comment when asked several questions by CNN about its contract with the UK, how it prioritizes different markets with contracts based on “best reasonable efforts” and for details around its supply chains to the UK and EU.
When asked whether the agreement to prioritize the UK was a redacted part of its contract or included in any other legal document, BEIS said only that: “The UK Government has an agreement with AstraZeneca to supply 100 million doses of the University of Oxford/AstraZeneca vaccine, and have agreed delivery timescales for this.
“The detail of any commercial agreements between the UK government and AstraZeneca for the Oxford/AstraZeneca vaccine are commercially sensitive.”
But UK Health Secretary Matt Hancock suggested earlier this month to the LBC that the agreement was included in the contract.
“I wasn’t going to settle for a contract that allowed the Oxford vaccine to be delivered to others around the world before us. I was insisting we could keep all of the British public safe as my primary responsibility as the Health Secretary,” he said.
The issues of timing and prioritization are key in the European Union, which centralized its procurement of vaccines to ensure fair distribution among its member states. But its supply shortages – not just from AstraZeneca – meant its vaccination program got off to a slow start and is continuing on a start-stop basis in many countries.
A European Commission spokesperson did not want to comment on the UK’s contract with AstraZeneca, but said the commission regarded its own contract to include “binding orders and clear delivery quantities.”
“A best effort clause is there because the vaccine hadn’t yet been developed or authorized, and it was not clear whether AstraZeneca would produce a vaccine,” the spokesperson said.
“And now with the development of a vaccine which has now been authorized, there are clear delivery quantities, both for December of last year as well as the coming quarters for this year.”
Where does this leave AstraZeneca?
AstraZeneca’s contract with the EU is essentially the same as the UK’s in terms of language, with some differences to reflect that the EU was procuring on behalf of 27 nations, according to David Greene, a senior partner at the law firm Edwin Coe, who has read the two redacted contracts, and has not seen the unredacted versions.
“There are many similarities between these two contracts, including the best reasonable efforts terms. It’s hardly surprising because they were made at the same time,” he said.
He explained that the term “Best Reasonable Efforts” was essentially an escape clause to offer some legal protection to AstraZeneca in the event it could not deliver to its agreed schedule.
“However, what they can’t do, on the face of it, is choose one contracting party over another. So they can’t say to the EU ‘I’m not going to deliver to you because I’m going to deliver to the UK,’ and similar. That’s always been the case.”