The UK became the first country to begin administering a fully vetted and approved Covid-19 vaccine this week, starting with the elderly and frontline health workers.
Thousands of people have been given doses of the Pfizer/BioNTech vaccine since the rollout began on Tuesday. But some have questioned the speed at which it was approved by UK regulators as two health care workers – who both carried an adrenaline auto injector and had a history of allergic reactions – had allergic symptoms after receiving the shot.
CNN asked UK residents for their views, and whether they would take the vaccine once it was made available to them. Most said they were eager to have the injection and return to normal life after months separated from loved ones.
But others, including those with separate health issues, voiced doubts about the vaccine’s safety after its rapid development and approval process, and said they would prefer to wait to see any long-term effects.
‘Watching others die gives me flashbacks’

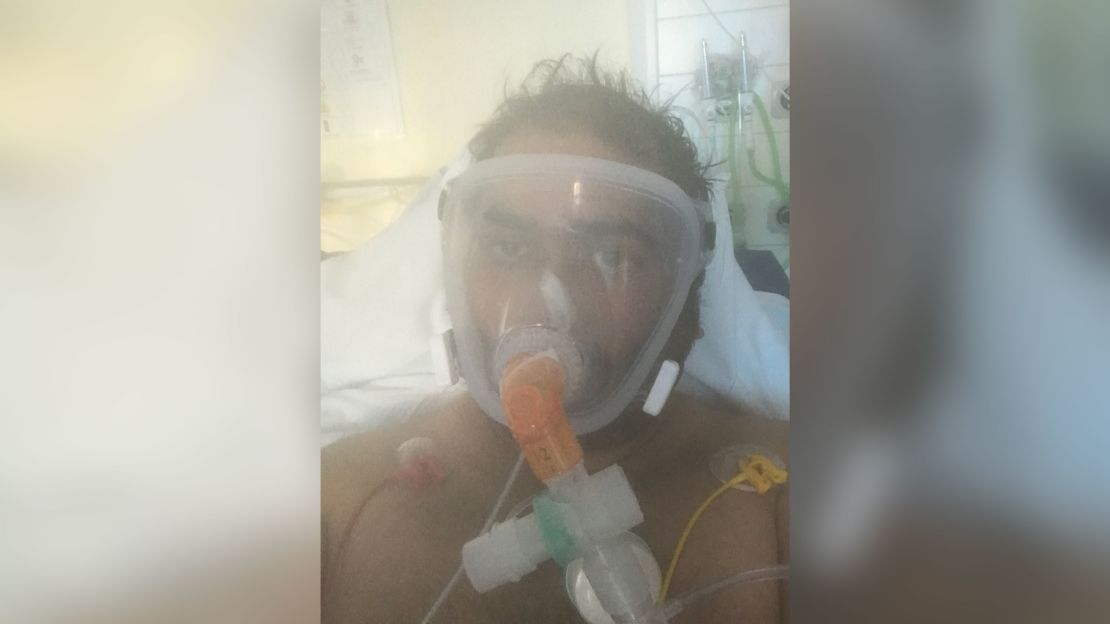
Hamza Ansari, a trauma doctor at the Royal Shrewsbury Hospital in western England, has more reason than most to be relieved that a vaccine is available. He nearly died after contracting Covid-19 while treating patients in March, and has since witnessed many deaths from the disease.
“I am extremely pleased and excited we finally have a vaccine and look forward to getting it,” he said. “This vaccine to me means a great deal, we are giving people a fighting chance.”
Ansari spent nine days in a high dependency unit on 100% oxygen for respiratory failure. He said he was back at work two weeks after he was discharged, despite not having fully recovered, because his team “needed all hands on the frontline.”
“Watching others die gives me flashbacks of what I went through,” he said. “It’s tough to watch, I’ve been through it and all we can do is guide them and support them, hoping they’ll make it out alive.”
He said the biggest concern was asymptomatic carriers who spread the virus without realizing it.
“Our goal is to prevent people from contracting the virus and keeping them out of hospital. This is a first step in a long journey,” he said.
John Swindells, from Lancashire, northern England, said it felt as though “life has been on hold this year,” with his daughter’s wedding in April, his son’s wedding on New Year’s Eve and his 40th wedding anniversary cruise all canceled. He said he was just waiting for the call to have the vaccine, “which we would have without any hesitation so that we can plan for the future.”
“Even a much smaller Christmas is bearable now there is light at the end of a long dark tunnel where we have spent virtually nine months stuck at home,” said the 69-year-old.
He said the approval of the vaccine was a “huge boost” and “gives us hope we can meet family and friends again” and take vacations.

But Bridget Jones, from Sevenoaks in Kent, said she would not have the vaccine because of her medical history of reacting to previous shots.
The 60-year-old said she was “NOT an anti-vaxxer” but explained: “I have had cancer twice, my immunity is low and I am worried what effect the vaccine will have because of this.”
After Wednesday’s news that two UK health workers had suffered allergic reactions to the shot, Jones added: “This hasn’t exactly improved my thoughts about taking the vaccine. In fact it has made me even less inclined to take it.”
Getting the shot
Meanwhile, Aiah Mbriwa, from Reading, west of London, said he couldn’t wait to have the vaccine before he was given his first dose on Wednesday.
The 41-year-old, a Sierra Leonean working in the UK’s health and social care sector while studying, traveled with colleagues and waited for more than two hours to get the shot. “Vaccination is my best bet so far and I am going for it without fear or hesitation,” he said.
Afterwards, Mbriwa said it all went well, except for some delays in the process, and he was pleased to have had the vaccine: “The injection itself was not painful to me … I have not experienced anything unusual or abnormal.”
He added: “I believe the beginning of anything is bound to have teething issues and with all personnel trying to be careful to get it all right first time, I cannot complain.”

Others are in no hurry. Jill Bunker, an American living in Manchester in northern England, said she wanted to know more about long-term effects. “I just have a little bit of hesitation,” she said. “I’m okay with not going first.”
She said she would like to see the effects on people in six months to a year, and learn how long the protection lasted.
“People talked about a lot at the beginning of the pandemic about how a vaccine takes years and years and years to be developed. And then all of a sudden, we have one and we’re supposed to believe that it’s safe.”
The 31-year-old said many women she knew were worried about the effects while pregnant, breastfeeding or trying to get pregnant.
UK regulators have said that until more evidence is available, people who are pregnant, breastfeeding or planning to get pregnant within three months should delay their vaccination.
Stephen Leadbetter, from Staffordshire in England’s West Midlands, said he probably wouldn’t take the vaccine if he was offered it this week.
“I am worried about side-effects of the vaccine at present, as I don’t believe that the UK’s express approval of the first available Western vaccine has been carried out with due scientific diligence,” said the 29-year-old.
“Vaccination will ultimately protect my family, but I am worried that there may be a price months down the line that may not be foreseen with such an expedient review and approval process.”
Alan Duncan, 64, said only one person he knew was in a hurry to get the vaccine, and that was because they have heart problems. “My main concern is that never in the history of vaccine development has a safe one been found in a matter of months,” he said.
“Many vaccines have had to be extensively refined and tested before mass use.
“Vaccines are not magic bullets, many of them are not 100% effective and all can have side-effects, some serious.”
As the UK announced the approval of the Pfizer/BioNTech vaccine on December 2, the country’s health department said the decision “follows months of rigorous clinical trials and a thorough analysis of the data” by the Medicines and Healthcare products Regulatory Agency, which had “concluded that the vaccine has met its strict standards of safety, quality and effectiveness.”
Loved ones at risk
Claire, from London, said she would have the vaccine and – more importantly – she wanted her elderly and clinically vulnerable mother to take it too.
“I would like to see her again,” said Claire. “I don’t have any reservations.”
CNN agreed to identify Claire by her first name only, given her family’s request for privacy during a difficult year.
The 54-year-old said her father died in a care home from Alzheimer’s disease this year and she hasn’t seen her mother since his funeral in early summer.
“We didn’t see dad after early March as the home went into lockdown before the actual lockdown. We weren’t there at the end because he decided to leave us without warning,” she said.
She said she hoped her father was unaware that his family was not there when he died.

Vincent McCabe, from Bognor Regis in West Sussex, has been caring for his younger brother after he was severely injured in a motorcycle accident last December.
He said he had been taking “every possible measure to protect him from getting Covid 19,” including using PPE at all times.
“I will get vaccinated as soon as it is made available to me, because it is the responsible thing to do, and to further protect my younger brother from getting Covid from me.”
McCabe said he is not at the front of the line for the vaccine, but will have it once it is available for the 60-65 age group.
“A vaccine might cause unwanted side-effects in a small number of people, but we know that Covid kills,” he said.
Matt Grant, 39, is an American working in London who usually travels to Philadelphia every three weeks to see his children, aged 8 and 10. The pandemic and mandated quarantines on both sides of the Atlantic have made it much harder, and he has gone several months at a time without seeing them.
“It has been terrible,” Grant said, adding that he had managed to travel for Thanksgiving and was currently in quarantine in the UK.

Grant said he welcomed the vaccine and saw it as a “great day,” but he said he worried that airlines could impose bans on people who have not been vaccinated. “If this is done before it is made available to everyone, it has the potential to limit my ability to travel back and forth to see my children,” he said.
Bridget Schiller, also from Reading, said she had “a terrible phobia of needles” but also wanted to travel and see her parents, who she hasn’t seen in a year.
“I normally spend Christmas with them in Florida but this year I’m in Reading and they’re alone in Florida,” she said.
“I want to hug them, help them set up the smart TV they got on Black Friday and just run errands for them.
“So phobia or not I will be getting the vaccine.”