Story highlights
NEW: Expert: Results of trial indicate a "unique breakthrough" in combating Ebola
"This is an extremely promising development," says head of World Health Organization
The vaccine has been tested in Guinea since March
A newly developed vaccine against the deadly Ebola virus is “highly effective” and could help prevent its spread in the current and future outbreaks, the World Health Organization said Friday.
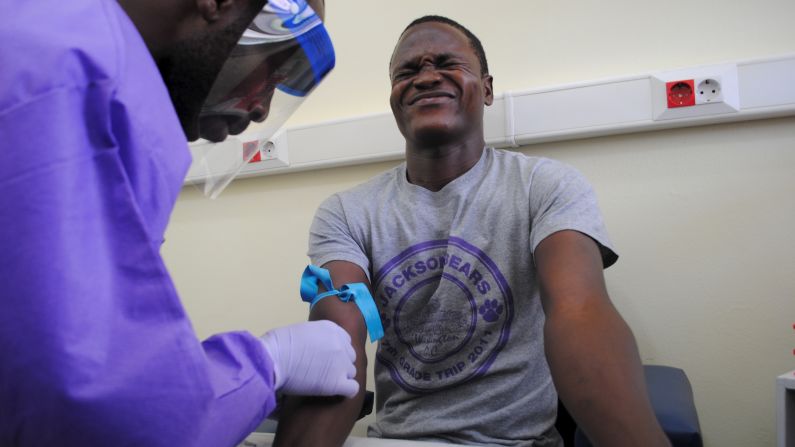
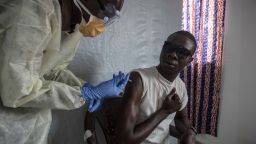
Trials of the single-dose VSV-EBOV vaccine began in March in Guinea – one of three West African nations at the center of the recent outbreak – and have shown such promise that this week it was decided to extend immediate vaccination to “all people at risk” after close contact with an infected person, a WHO statement said.
“This is an extremely promising development,” said Dr. Margaret Chan, the body’s director-general.
“The credit goes to the Guinean government, the people living in the communities and our partners in this project. An effective vaccine will be another very important tool for both current and future Ebola outbreaks.”
More research is needed, but the results so far on this trial show 100% efficacy.
It will take weeks at the least, and possibly a couple of months, for more supply to be made, according to Chan.
Researchers have been using a “ring” strategy – based on that used in smallpox eradication in the 1970s – to test the vaccine’s effectiveness.
“The premise is that by vaccinating all people who have come into contact with an infected person you create a protective ‘ring’ and stop the virus from spreading further,” said John-Arne Rottingen of the Norwegian Institute of Public Health, which has been involved in implementing the trial.
Relatives, co-workers, health workers get jab
To date, more than 4,000 close contacts of almost 100 Ebola patients, including family members, neighbors and co-workers, have voluntarily participated in the trial, the WHO statement said.
Until this week, half were vaccinated three weeks after the identification of an infected patient and others straight away, to allow for comparison of the results. The randomization was stopped on Sunday “to allow for all people at risk to receive the vaccine immediately, and to minimize the time necessary to gather more conclusive evidence needed for eventual licensure of the product,” the WHO said.
The trial will now include 13- to 17-year-olds, and possibly children from age 6, on the basis of new evidence of the vaccine’s safety, it added.
The vaccine has also been given to 1,200 front-line health workers, laboratory staff, cleaning staff and burial teams, Doctors Without Borders said.
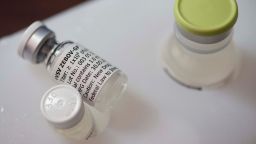
The VSV-EBOV vaccine was developed by the Public Health Agency of Canada and licensed to Merck and NewLink.
The Guinea trial is being implemented by the Guinean authorities, the WHO, Doctors Without Borders and the Norwegian Institute of Public Health, with support from international and national organizations.
Medical journal The Lancet published the phase three trial’s interim results Friday.
Dr. Bertrand Draguez, who’s been leading Doctors Without Borders efforts to find new tools to combat Ebola, said more data was needed – for example, on how soon protection kicks in after vaccination and how long it lasts – but that the results suggest a “unique breakthrough” in fighting the disease.
“Even if the sample size is quite small and more research and analysis is needed, the enormity of the public health emergency should lead us to continue using this vaccine right now to protect those who might get exposed to the disease: contacts of infected patients and front-line workers,” he said.
Because the virus is concentrated in “hot spots” across the region, it makes more sense to focus on vaccinating those close to infected patients and front-line workers than to embark on a mass vaccination campaign, he said.
Ebola virus: Nine things to know about the killer disease
Jesse L. Goodman, professor of medicine at Georgetown University Medical Center in Washington, said the results published in The Lancet provided “exciting preliminary evidence” that the vaccine is likely to be effective but also cautioned that further analysis was needed.
“Nonetheless, the degree of protection reported seems convincing,” he said.
The concerted effort to find a vaccine reflects the severity of the crisis presented by Ebola, spread through contact with body fluids from an infected person or contaminated objects from infected persons. Other vaccines are also beingtested.
Fighting Ebola with HIV drugs gets big boost
WHO: Lowest weekly total in more than a year
There have been more than 11,000 reported deaths in the three worst-affected countries – Guinea, Sierra Leone and Liberia – since the epidemic took hold last year.
The number of new Ebola cases is now far below that of the outbreak’s peak, but it has remained stubbornly difficult to eradicate.
In pictures: The Ebola epidemic
On Sunday, the WHO reported seven Ebola cases were confirmed in the preceding week – four in Guinea and three in Sierra Leone.
“This is the lowest weekly total for over a year, and comes after 8 consecutive weeks during which case incidence had plateaued at between 20 and 30 cases per week,” the WHO said.
Two people in Liberia, including a 17-year-old in Nidonwin, have died of Ebola since the end of June, weeks after the WHO declared the nation free of the disease. At the time, though, officials warned outbreaks in Guinea and Sierra Leone ran the risk of bringing the virus back to Liberia, where more than 4,000 people died after contracting it.
All 33 contacts in Liberia who have been followed up since the latest infections there are two days from completing the 21-day period to be declared free of the disease.
CNN’s Debra Goldschmidt contributed to this report.