Story highlights
In two new studies, adult skin cells were used to make embryos
Stem cells can grow into any kind of tissue
Many future medical advances are hoped to arise from stem cells
For the first time, cloning technologies have been used to generate stem cells that are genetically matched toadult patients.
Fear not: No legitimate scientist is in the business of cloning humans. But cloned embryos can be used as a source for stem cells that match a patient and can produce any cell type in that person.
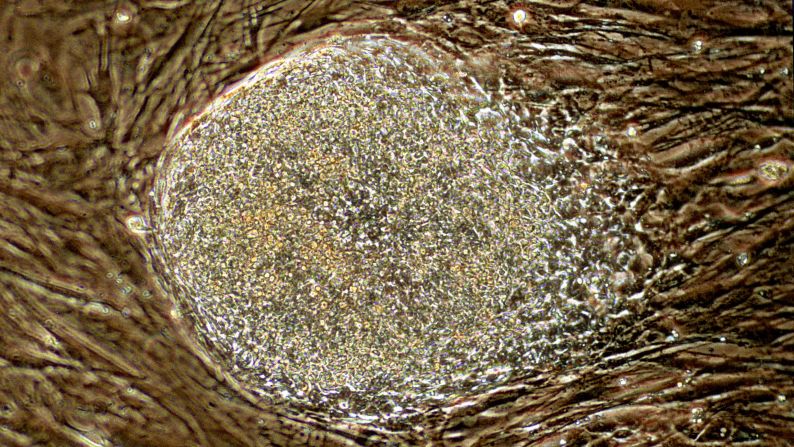
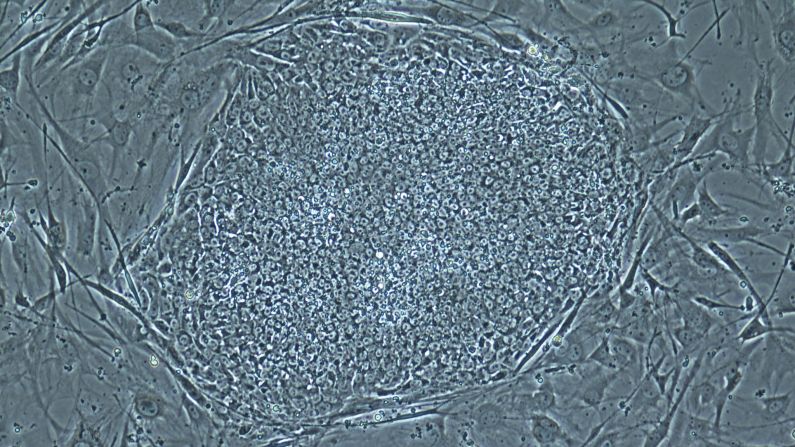
Researchers in two studies published this month have created human embryos for this purpose. Usually an embryo forms when sperm fertilizes egg; in this case, scientists put the nucleus of an adult skin cell inside an egg, and that reconstructed egg went through the initial stages of embryonic development.
“This is a dream that we’ve had for 15 years or so in the stem cell field,” said John Gearhart, director of the Institute for Regenerative Medicine at the University of Pennsylvania. Gearhart first proposed this approach for patient-specific stem cell generation in the 1990s but was not involved in the recent studies.
Stem cells have the potential to develop into any kind of tissue in the human body. From growing organs to treating diabetes, many future medical advances are hoped to arise from stem cells.
Scientists wrote in the journal Cell Stem Cell this month that they used skin cells from a man, 35, and another man, 75, to create stem cells from cloned embryos.
“We reaffirmed that it is possible to produce patient-specific stem cells using a nuclear transfer technology regardless of the patient’s age,” said co-lead author Young Gie Chung at the CHA Stem Cell Institute in Seoul, South Korea.
On Monday, an independent group led by scientists at the New York Stem Cell Foundation Research Institute published results in Nature using a similar approach. They used skin cells from a 32-year-old woman with Type 1 diabetes to generate stem cells matched to her.
Both new reports follow the groundbreaking research published last year by Shoukhrat Mitalipov and colleagues at Oregon Health & Science University in the journal Cell. In that study, researchers produced cloned embryos and stem cells using skin cells from a fetus and an 8-month-old baby.
“It’s a remarkable process that gives us these master cells, these stems cells that are essentially the seeds for all of the tissues in our bodies,” said George Daley, director of the Stem Cell Transplantation Program at Boston Children’s Hospital, who was not involved in the recent studies. “That’s why it’s so important for medical research.”
A brief history of stem cells
The first developments in the field of stem cell research used leftover embryos created by the union of sperm and egg from in vitro fertilization.
But embryonic stem cell research is controversial because to use the stem cells for developing medical treatments, the embryo is destroyed. Embryos have the potential to develop into a fully formed human, bringing up ethical questions.
Scientists later realized that it’s not necessary to use embryos to obtain stem cells that match patients. Shinya Yamanaka won the 2012 Nobel Prize for Physiology or Medicine for discovering how to make “induced pluripotent stem cells,” or IPS cells.
IPS cells are made by inserting genes to “turn back the clock” on mature cells that already have specific functions.It doesn’t matter what the cell was before; it can now be reprogrammed as any kind of cell researchers want.
Why, then, would researchers bother to make stem cells using cloning, which requires human eggs and the creation of embryos?
“People have made patient-matched stem cells using IPS methods,” said Dieter Egli, senior author on the Nature paper that could have implications for diabetes treatment and researcher at the New York Stem Cell Foundation. “But it is not clear in the U.S. at the very least, and also elsewhere, how and if these are going to be translated into people.”
Egli points out that there have been some reports that IPS cells may have shortcomings when converted into specific cell types, and that stem cells produced by cloning may be better.
But which stem cell type is better and safer – the IPS cells or cells from cloned embryos? That is still an open question. To settle it, there would need to be a comparison of the two stem cell types generated using the DNA of the same person.
Gearhart doesn’t see the cloning method replacing the use of IPS cells, which are not controversial and don’t require that women donate their eggs.
“As we learn more about the reprogramming process that normally occurs in the egg following fertilization, we can use that information to produce better IPS cells,” Gearhart said.
The process of extracting eggs is complex and expensive; having enough supply is a “serious concern,” Daley said.
“The more we learn about reprogramming, the more I think IPS will be the one of choice,” Gearhart said.
Making stem cells by cloning
An embryo, the earliest stage of human development, is a cluster of cells smaller than the period at the end of this sentence.
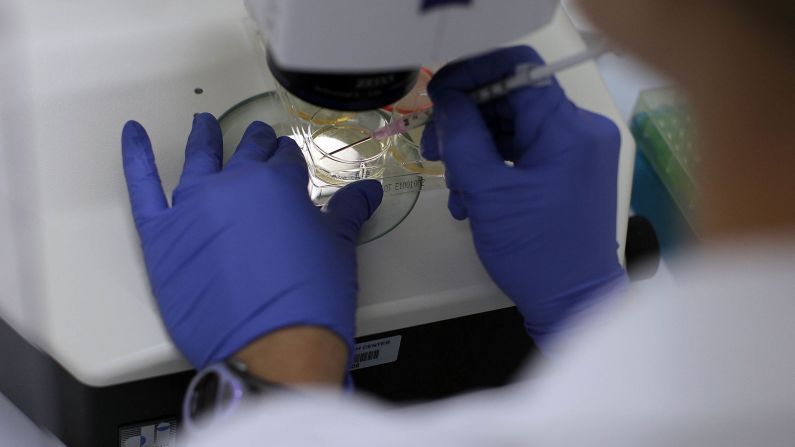
To make a cloned embryo, scientists use equipment analogous to “like a half-million-dollar video game,” Daley said.
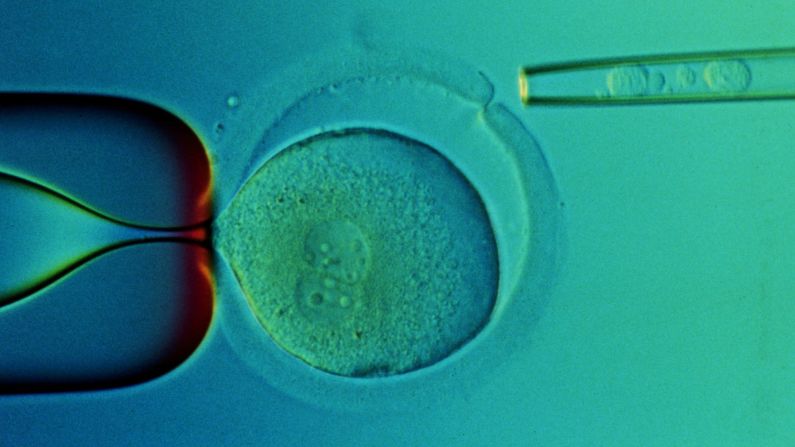
Researchers perform surgery on eggs with needles that are the 10th of the size of a human hair. They use joysticks to manipulate the tiny equipment, spearing the egg, removing its DNA and then transferring the nucleus of a skin cell into the egg.
“That process, which is called nuclear transfer, sets in motion this remarkable process of early human development,” Daley said. “We trick this reconstructed egg into thinking it’s been fertilized.”
Chung’s group – which led the Cell Stem Cell study – used this cloning method that Mitalipov had pioneered to get two stem cell lines out of 77 eggs. “It seems that the quality of oocyte (egg) plays a pivotal role,” Chung said.
Egli and colleagues had their own spin on the cloning process, amending it so that it happens in a more controlled way. Their study in Nature used electricity in combination with chemicals, and manipulating the calcium concentration, to improve the procedure. They generated stem cells specific to the diabetic patient who had donated skin cells; the eggs came from donors.
This group got four cell lines from 71 eggs, said research assistant Lydia Mailander. The average egg donation is 14 to 15 oocytes. Researchers estimate it would take two such donations to get one stem cell line.
In mice, Egli and colleagues showed in a separate study that the cloned stem cells from the diabetic patient mature into glucose-responsive beta cells, which secrete insulin into the bloodstream of the animals.
Further considerations
It’s not clear that there are enough eggs in supply to support a large-scale production of stem cells this way, experts said.
For the near-term research purposes, there seem to be enough eggs available for stem cell cloning: About 10,000 egg donor cycles occur annually in the United States, Egli said.
But egg donation for research is generally low, Gearhart said. According to the American Diabetes Association, about 1 million Americans have Type 1 diabetes, the target population for a potential stem cell therapy.
The cloning method takes a few weeks, and is not significantly faster than generating IPS cells, Egli said.
Including compensation to the woman, an egg donation cycle costs about $14,000, Egli said.
“It may not at all be more expensive (than IPS) if the cells that we make are actually better,” he said. “That’s why it’s important to do these comparisons.”
Such a comparison would be interesting, said Daley, but the advantages would have to be considerable to beat out IPS, which is “much more efficient and less ethically contentious that Yamanaka (the Nobel winner) taught us.”
Still, from a research perspective, the cloning method could help scientists interested in understanding how an egg reprograms the cell, Daley said. It could help answer: “How does an egg reset the whole identity of an adult cell back to an embryonic state?”
Stem cell cloning research might, in this way, teach scientists how to make stem cells more efficiently, he said, and optimize them for medical applications.
Tell us your story!
Tell us your story!
We love to hear from our audience. Follow @CNNHealth on Twitter and Facebook for the latest health news and let us know what we’re missing.
How about making a clone?
Mitalipov told CNN in 2013 that the embryos created in his study, from skin cells and eggs, would not grow babies. That would have required additional technology, and it wasn’t part of the study.
But the same basic “nuclear transfer” principle used in Mitalipov’s, Chung’s and Egli’s studies was used create Dolly the sheep, the first mammal clone.
In Dolly’s case, an embryo created by nuclear transfer was transplanted to a ewe, which gave birth to a sheep.
In the case of human embryos generated in these stem cell experiments, “it would be dangerous and unethical to attempt to transfer them into a uterus,” Daley said.