Story highlights
- A new drug, Belviq, is available to overweight and obese patients
- The drug received FDA approval nearly a year ago
- It works by activating a serotonin receptor in the brain
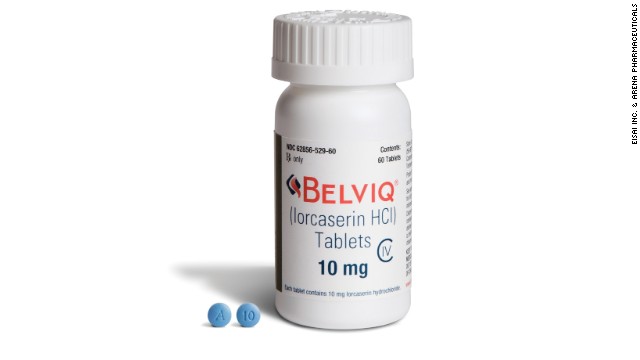
Nearly a year after receiving approval from the U.S. Food and Drug Administration, a new obesity drug will be available to certain patients by prescription, starting Tuesday.
The FDA approved Belviq, an oral medication, in June 2012. The delay in availability was in part because the Drug Enforcement Administration needed to review the drug.
People with a body mass index of more than 30 or a BMI of 27 with at least one weight-related condition, such as hypertension or Type 2 diabetes, are eligible for a prescription.
Belviq, as the FDA pointed out in its statement announcing approval last year, is intended "as an addition to a reduced-calorie diet and exercise."
Belviq, or lorcaserin hydrochloride, works by activating a serotonin receptor in the brain, which may help a person eat less and feel full after eating smaller amounts of food, according to the FDA.
Belviq received approval about the same time as another weight-loss pill, Qsymia, which hit the market in September.
Weight loss with both drugs is modest. Patients in clinical trials went from an average 227 pounds to 204 pounds on Qsymia; on Belviq, the average weight dropped from 220 to 207.
In trials, 47% of patients without Type 2 diabetes lost at least 5% of their body weight on Belviq, according to the FDA. By comparison, 23% of patients treated with a placebo lost at least 5% of their weight. In people with Type 2 diabetes, 38% of patients on Belviq lost at least 5% of their body weight, compared with 16% on a placebo.
At the time of approval, the FDA said Belviq's manufacturer, Arena Pharmaceuticals, would be required to conduct six post-marketing studies, including a long-term cardiovascular trial to assess the risk of heart attack and stroke.
The most common side effects of Belviq in nondiabetic patients included headache, dizziness and fatigue, according to the FDA. In diabetic patients, side effects included pain and low blood sugar.
In addition, some people taking medicines such as Belviq have reported heart valve problems, according to Eisai Inc., which markets the drug.
"It is not known if Belviq changes your risk of heart problems or of stroke, or death due to heart problems or stroke," according to the drug's website.