As waves of new coronavirus variants circulate the globe, one thing has become clear: human immunity against the virus fades over time.
To maintain durable protection against the virus that causes Covid-19, scientists are working around the clock to develop next-generation vaccines. But some of the nuances around why and how immunity against Covid-19 fades remain a mystery.
The steepest drops in immunity – which come about four to five months after vaccination and up to eight months after infection, but can vary – are against Covid-19 symptoms, getting infected and getting sick. Protection against severe outcomes, hospitalization and death remains much higher for a longer period of time, but even this decays to some degree, especially for the elderly and those with compromised immune function.
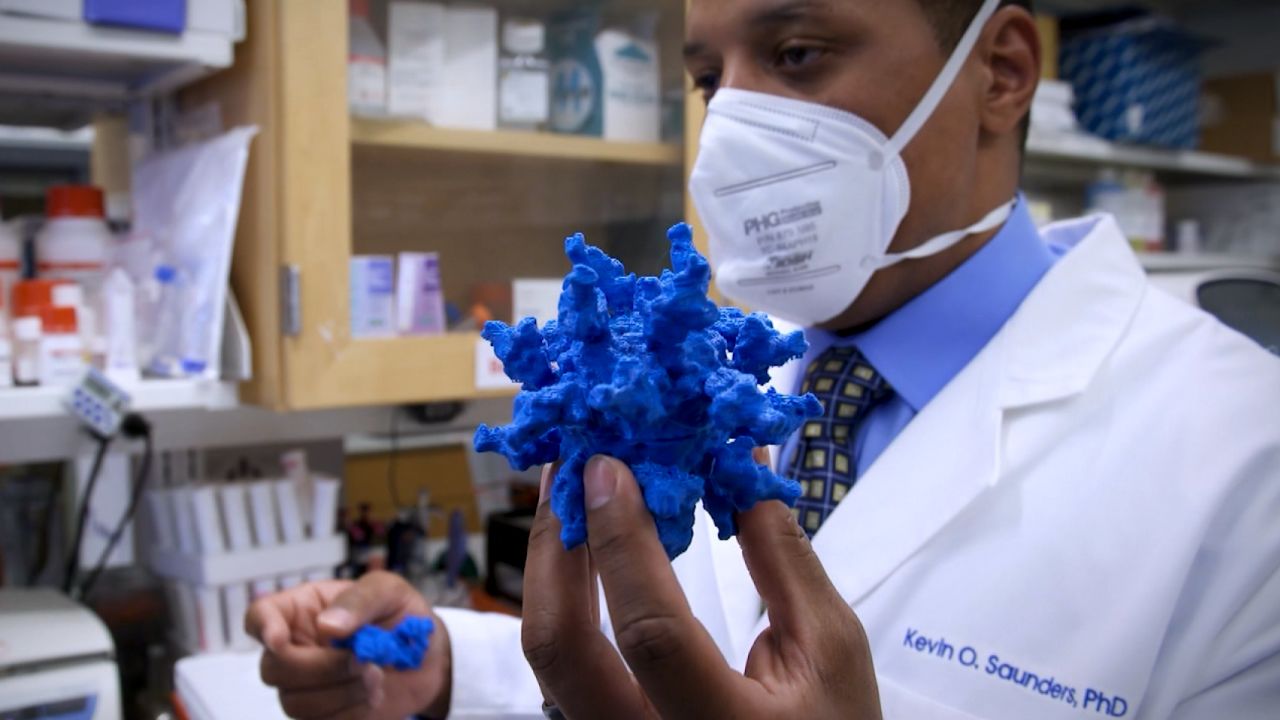
Since the early days of the pandemic, scientists have known that the coronavirus carries a structure called the spike protein, and it uses this crown of spikes to get into the cells it attacks. Our Covid-19 vaccines create antibodies to these spike proteins that bind to the docking sites on the virus, blocking them from infecting our cells.
Yet our safety net against the virus is wearing thin, in part because the virus is changing like a fugitive donning a disguise – picking up mutations that change the shape of its spikes in ways that make it less recognizable to our immune system.
But there’s another piece of the immunity puzzle that scientists are urgently trying to solve, and that is whether some of this drop off in our protection may be a result of the mRNA technology used to build some Covid-19 vaccines, such as those developed by Moderna and Pfizer/BioNTech, which were the first in the world to use this platform.
“Some vaccine platforms give a very high degree of protection but the durability isn’t very long,” said Dr. Anthony Fauci, director of the National Institutes of Allergy and Infectious Diseases in an interview with CNN.
Fauci said that the mRNA platform may be one of those.
In clinical trials, the new mRNA vaccines have proven to be astonishingly good at protecting people against illness, hospitalizations and deaths, at least in the short term. Fauci said mRNA vaccines have other advantages, too. It’s relatively fast and easy to redesign them to better protect against new variants, for example.
“We got a really great platform with mRNA,” Fauci said. “But let’s try to be better. Because our experience, maybe it’s peculiar to coronavirus, but I doubt it, is that the durability of the response you can be better on.”
‘We’ve got to get better platforms’
To be fair, Fauci said we won’t know how long immunity induced by these kinds of vaccines may last until mRNA is used to make vaccines against a different type of pathogen, perhaps one that doesn’t change as much as SARS-CoV-2, the virus that causes Covid-19.
Definitive answers may be years away.
In the meantime, he said, we can’t wait. We need to improve the vaccines if we’re going to keep Covid-19 at bay.
“We have very good vaccines, but we’ve got to get better platforms and immunogens, maybe with adjuvants that allow us to have a greater durability of protection,” Fauci said. Adjuvants are extra ingredients in vaccines that help them work better.
Other experts agree.
Deepta Bhattacharya heads a lab at the University of Arizona where he studies the lifespan of plasma cells, a type of long-lasting cell that makes protective antibodies. He’s also interested in how various vaccine technologies influence the persistence of these cells in our bodies.
What we can tell after more than a year of experience with the mRNA vaccines is that their protection starts high but seems to fade more quickly than the immunity that remains after a Covid-19 infection, according to Bhattacharya.
“There have been a few side-by-side studies that have compared the mRNA vaccines to infection-induced immunity, and it seems like it slips a little bit faster than that,” Bhattacharya said.
Though he cautioned that protection after an infection varies greatly from person to person just because everyone’s immune system is a little different. There’s no good way to know, right now, how well any particular person’s immune system responds to a vaccine, which is why it’s important to be vaccinated, even if you’ve already had Covid-19.
He added that when comparing the performance of the mRNA vaccines to adenoviral vector vaccines, such as those developed by AstraZeneca and Johnson & Johnson, people initially make a lot more antibodies after vaccination with an mRNA vaccine, but these levels seem to fall pretty steeply by around the six-month mark. Adenovirus vaccines use another virus as a Trojan horse to sneak instructions for making the spike proteins into cells.
With the adenoviral vector vaccines, antibody levels don’t seem to climb as high initially as they do with mRNA vaccines, but they do seem to persist for longer periods at these lower levels, pointing to some difference in the body’s response to them that we don’t fully understand.
In a large study of more than 35,000 health care workers in the United Kingdom, compared to those who were unvaccinated, those who had two doses of the Pfizer/BioNTech mRNA vaccine were about 85% less likely to get a Covid-19 infection, through about two and a half months after their second dose. But by six and a half months, that protection against infection had fallen to about 51%.
The follow up period for the study was between December 7, 2020, when the vaccines were first given to healthcare workers in the UK to Sept. 21, 2021, so it doesn’t include Omicron infections.
Health care workers with two doses of the Astrazeneca adenoviral vector vaccine were about 58% less likely to get a Covid-19 infection compared to those who were unvaccinated, through about two and a half months after vaccination, but the effectiveness of that vaccine appeared to increase over time, cutting the risk of infection by more than 70% about seven months after a second dose.
Health care workers who caught a Covid-19 infection, most of them happening in March 2020, before the era of vaccines, were initially about 86% less likely to be reinfected, and that protection lasted up to a year. After a year, it dropped to about 69% in workers who were unvaccinated, which was still better than the protection from mRNA vaccines alone.
Workers who had caught Covid-19 and went on to be vaccinated had the best protection of all, more than a 90% lower risk of getting Covid-19 again, and that combined protection stayed high for the duration of the study, which was more than 9 months.
This evidence and other studies, said Bhattachayra, suggests our immunity against Covid can be tweaked to make it last longer.
“I do think it’s fair to ask more of our vaccines and that they sort of maintain that protection for longer,” Bhattachayra said.
“I think there’s still very clearly room for improvement because there are certain vaccines that do better” in terms of their durability, he said. “There’s no question about that.”
Other vaccines have needed improvements to help them last longer
Starting at two months of age, doctors recommend that babies get a vaccine against Haemophilus influenzae, or Hib, a common bacteria that can cause serious infections if it invades the lungs, blood or brain. These bacteria are coated with chains of sugars, or polysaccharides, that help mask them to our immune systems.
In the 1980s, scientists figured out that you could use those sugar chains to build a vaccine to protect children from serious infections.
“The initial Hib vaccine was a polysaccharide vaccine, but it did not induce long-lived antibody levels, so we don’t even use it now,” said Dr. Gregory Poland, an infectious disease expert who studies how the immune system responds to vaccines at the Mayo Clinic in Minnesota.
Today’s Hib vaccine still contains the sugar chains, but they are linked to protein pieces that stimulate a different part of the immune system to better remember the bacteria. It’s called a protein conjugate vaccine.
Another example of a vaccine that didn’t ultimately provide long-lasting immunity was the pneumococcal vaccine for pneumonia. It, too, started life as a polysaccharide vaccine, but was changed to a protein conjugate after researchers determined that change could extend its protection.
Some vaccines use extra ingredients, called adjuvants, to hyperstimulate the immune system, which increases the strength of the protection people get from them. These kinds of vaccines are often used for older adults and others whose immune systems need an extra kick in the pants, so to speak, to work.
Certain vaccines inherently do this, just because of the way they are designed, Fauci said, and the nanoparticles being built into some experimental vaccines are an example of this.
Fauci added that he’s not sure why the immune response triggered by mRNA vaccines may not be longer lasting. He has some theories, though.
One of the early failures in developing the mRNA technology was that when the chains of molecules called nucleic acids were injected into animals, they triggered an immune response too quickly. The animals got sick and their immune systems destroyed these chains – or instructions – before cells could read them and build the proteins they coded for.
One breakthrough in turning these instructions into vaccines was that the scientists who developed them figured out how to make a chemical change to the mRNA to hide it from the immune system until it could get inside cells, thus, reducing the risk of getting sick.
“They modified the molecule to remove the inflammatory aspect of it, to allow it to be used as a vaccine, that possibly–and I underlined 15 times, possibly–could be reason why,” Fauci said.
“Maybe if we use this mRNA, but add a different adjuvant with it, you might get a really good response, the best of both worlds, you might get the real advantage of an mRNA together with a bit more durability, if you add to it an adjuvant as opposed to having the molecule itself be inherently adjuvant.”
Bhattachyra has another theory about why the mRNA platform may not be lasting as long.
He said these vaccines instruct cells to build spike proteins from the virus and then display them on their surfaces, where they can be seen by the immune system.
But cells are giant compared to viruses – about 100 times larger, he said, and viruses pack about 25 spike protein trimers onto their smaller surface, making them pretty densely packed. A trimer is a type of chemical compound or molecule that has three pieces.
“I don’t know what the density of spike proteins is on a cell; it may not be as high as what it is on a virus, for instance,” Bhattachyra said. No one really knows what the spike-expressing cells look like and how closely they resemble the virus they’re targeting.
“It could be that the spacing is pretty infrequent and you’re just not getting the level of activation that you would want,” he said, adding “that’s pure speculation.”
Planning for the future
The United States is at a point in the pandemic now where health officials are grappling with the fact that to maintain immunity against Covid-19 in the community, the nation will either need to administer booster shots on a regular – or possibly annual – basis, or will need to rollout an entirely new vaccine altogether.
All vaccines have strengths and weaknesses – but some of the nation’s leading vaccine experts argue that more research is needed into the durability of the currently used Covid-19 vaccines as a potential weakness, as vaccine-induced immunity can decline within four to six months.
For instance, during the recent Omicron wave in the United States, the protection that vaccine boosters provide fell more than four months later from more than 90% to around 66% for protection against emergency room visits for Covid-19 and 78% against hospitalizations, Dr. Peter Hotez, CNN medical analyst and virologist and co-director of Texas Children’s Hospital Center for Vaccine Development, told CNN.
“The big unknown is this: How much of that decline is due to something quirky because of the Omicron variant? Or, is this a weakness in the technology and it’s not holding up? And it’s very hard to sort out,” Hotez said. “All vaccines have strengths and weaknesses, and it may be that for mRNA that it does not produce durable protection. It could be that you go in, use mRNA vaccines to rapidly immunize a population, stabilize it, but then over time, you’re going to have to come in with a heterologous boost that’s a different technology.”
Hotez, whose lab has developed a Covid-19 vaccine called Corbevax, said that the White House should convene vaccine experts in a special meeting to “pin down” whether the technology has that weakness and what it means for future strategies.
In an effort to find the answer to maintaining durable protection against Covid-19, several research groups are working to develop so-called “next-generation vaccines” that aim to induce longer-lasting protection and even “pan-coronavirus” vaccines, ones that offer protection against multiple variants of the coronavirus that causes Covid-19.
“How do you make the Covid responses induced by vaccines more long-lasting? How do you make this process of inducing long-lived plasma cells more efficient? That’s the name of the game right now,” said Dr. Barton Haynes, director of the Duke Human Vaccine Institute.
“There are a number of groups working on mRNAs for the next generation vaccines full well aware that, for those vaccines to be more long-lasting, breakthroughs need to be made,” he said.
Haynes is also working on a different type of vaccine — a nanoparticle containing fragments of the coronavirus’ spike protein. This vaccine also includes an ingredient that enhances the immune response, known as an adjuvant.
A key long-term goal is to create a more universal vaccine that can work against new variants of this coronavirus, plus others that cause common colds and even ones we haven’t identified yet. The protein-based vaccine is one of several that target conserved sites on the spike protein – ones that don’t mutate, lest they hinder their ability to infect human cells.
And Haynes said research in monkeys appears to show it does a better job than mRNA vaccines at generating those kinds of antibodies that provide broader coverage, as well. This may be due to a combination of how well the adjuvant works to stimulate the immune system, and perhaps the design of the nanoparticle itself, which almost looks like a virus, he added.
Regardless of the vaccine platform, “we’re all going after formulations that will induce durable, long-lasting antibody and other types of T-cell immunity.”
So far during the coronavirus pandemic, vaccine protection has been measured by the presence of antibodies in the blood. Antibodies are proteins made by the immune system to help fight infections. But there is more to the human immune system than just antibodies.
The immune system involves a host of players, including B cells, which produce antibodies, and T cells, which target infected cells during an infection – and T cells are often part of emerging discussions around vaccine durability.
A study published in the journal Cell in January showed just how much of a role the other parts of the immune system play in the durability of protection following Covid-19 vaccination.
The study found that, among 96 vaccinated adults, even though antibody levels decreased against coronavirus variants, the T cells induced by various types of Covid-19 vaccines – the Moderna, Pfizer/BioNTech, Johnson & Johnson and Novavax vaccines – were able to recognize coronavirus variants, including Omicron, even though the vaccines were developed based on the original coronavirus.
“The important thing for our study was we collected all those samples at the same place with the same techniques and ran all the experiments head-to-head so it was really a fair head-to-head test,” said Shane Crotty, virologist and professor at La Jolla Institute for Immunology, who was an author of the study.
Plus, those vaccines were developed using different technologies. Moderna and Pfizer/BioNTech are mRNA vaccines. Johnson & Johnson is a viral vector vaccine. Novavax, which is not yet authorized for emergency use in the United States, is a protein-based vaccine.
“The mRNA vaccines, both Moderna and Pfizer, generated these four categories: antibodies, memory B cells, helper T cells and killer T cells. Overall, the mRNA vaccines generated the best of all four of those,” Crotty said, adding that among people who were vaccinated with Johnson & Johnson, generally there appeared to be less of all four and Novavax appeared to generate somewhat less memory B cells and significantly less killer T cells.
“So, there were some different mixes there,” Crotty said. “Our immunological data are generally consistent with the vaccine efficacy data that’s out there from the clinical trials and real-world studies that in general, the mRNA vaccines are better than J&J in terms of protection from infection but also protection from hospitalization, with Novavax being somewhere in between, but doing quite well.”
Many experts seem to agree that discussions around future Covid-19 vaccination strategies should hinge on what exactly the goal of the vaccines are – to prevent the spread of the coronavirus or to keep people out of the hospital.
“So if your goal is to prevent any new infections in our society, then yeah, we’re going to have to keep boosting, because our antibody levels are going to decline – no matter what kind of vaccine we get,” Jen Gommerman, professor and acting chair in the Department of Immunology at the University of Toronto, told CNN.
She added that, among the vaccines authorized for emergency use in the United States, the rate of decline for the Johnson & Johnson vaccine is a bit lower than for Moderna’s and Pfizer/BioNTech’s mRNA vaccines. However, the peak level of protection that the Johnson & Johnson vaccine providers is lower than the peak for the mRNA vaccines.
“So, the raw amount of antibody in our serum is going down to a level where you will be susceptible to infection and the only way to get those antibodies back up quickly, is to either get infected or get boosted,” Gommerman said.
“However, if we consider efficacy against hospitalization and severe disease, the data coming out show that three doses of the mRNA vaccine confirm excellent protection against hospitalization, ventilation and death,” she said. “For me, personally, I would get a fourth dose if I knew it would serve public health reasons. But for me personally, I don’t feel I need a fourth dose to protect myself against severe disease, hospitalization or death.”
‘Many more questions still need answers’
There are still many questions left to answer about Covid-19 vaccines and immunology, John Wherry, director of the Institute for Immunology at the University of Pennsylvania’s Perelman School of Medicine, wrote in an email to CNN.
Those questions include: How long do memory B cells and memory T cells last? How do the vaccines containing the original coronavirus, identified in Wuhan, China, induce effective immune memory against all the variants so far? What immune mechanisms provide protection from infection versus protection from severe disease, hospitalization and death? Why do different people respond differently to these vaccines?
“These and many more questions still need answers if we are going to use this platform most effectively,” Wherry wrote. Such questions also need answers in the context of vaccine durability, especially as the durability of protection and the durability of immune responses themselves are related – but not the same, according to Wherry.
For the mRNA vaccines, “durability of protection is on par with other vaccine types from the analyses we have seen on the adenoviral platforms versus mRNA. Durability of immune responses – it’s been difficult to do really precise comparative studies longitudinally over a time frame relevant to answer this question,” Wherry wrote in his email.
Get CNN Health's weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
After all, the vaccines have only been around for about a year and a half, and answering questions around durability precisely can be founded by the introduction of booster shots and the incidence of breakthrough infections.
“This later point is relevant because it dramatically influences the durability to immune responses over time,” Wherry wrote about breakthrough infections. “The bottom line is that the data so far look very promising for durability of immune responses and protection from severe disease. Protection from mild disease is much more difficult for this virus and might only be achieved transiently when antibody levels are extremely high.”